Patents
Literature
102 results about "Rate dependent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
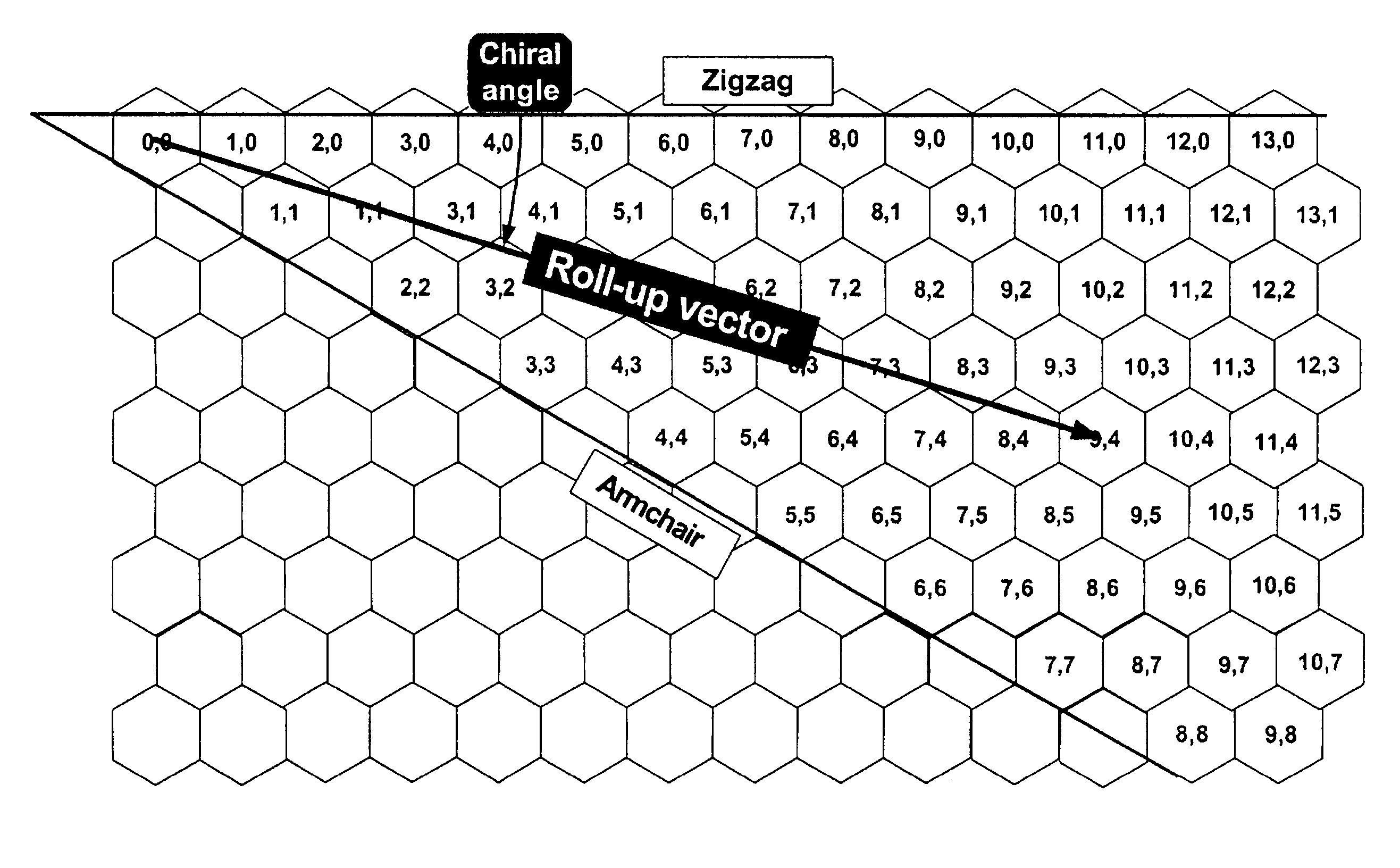
Method for separating single-wall carbon nanotubes and compositions thereof
The invention relates to a process for sorting and separating a mixture of (n, m) type single-wall carbon nanotubes according to (n, m) type. A mixture of (n, m) type single-wall carbon nanotubes is suspended such that the single-wall carbon nanotubes are individually dispersed. The nanotube suspension can be done in a surfactant-water solution and the surfactant surrounding the nanotubes keeps the nanotube isolated and from aggregating with other nanotubes. The nanotube suspension is acidified to protonate a fraction of the nanotubes. An electric field is applied and the protonated nanotubes migrate in the electric fields at different rates dependent on their (n, m) type. Fractions of nanotubes are collected at different fractionation times. The process of protonation, applying an electric field, and fractionation is repeated at increasingly higher pH to separated the (n, m) nanotube mixture into individual (n, m) nanotube fractions. The separation enables new electronic devices requiring selected (n, m) nanotube types.
Owner:RICE UNIV
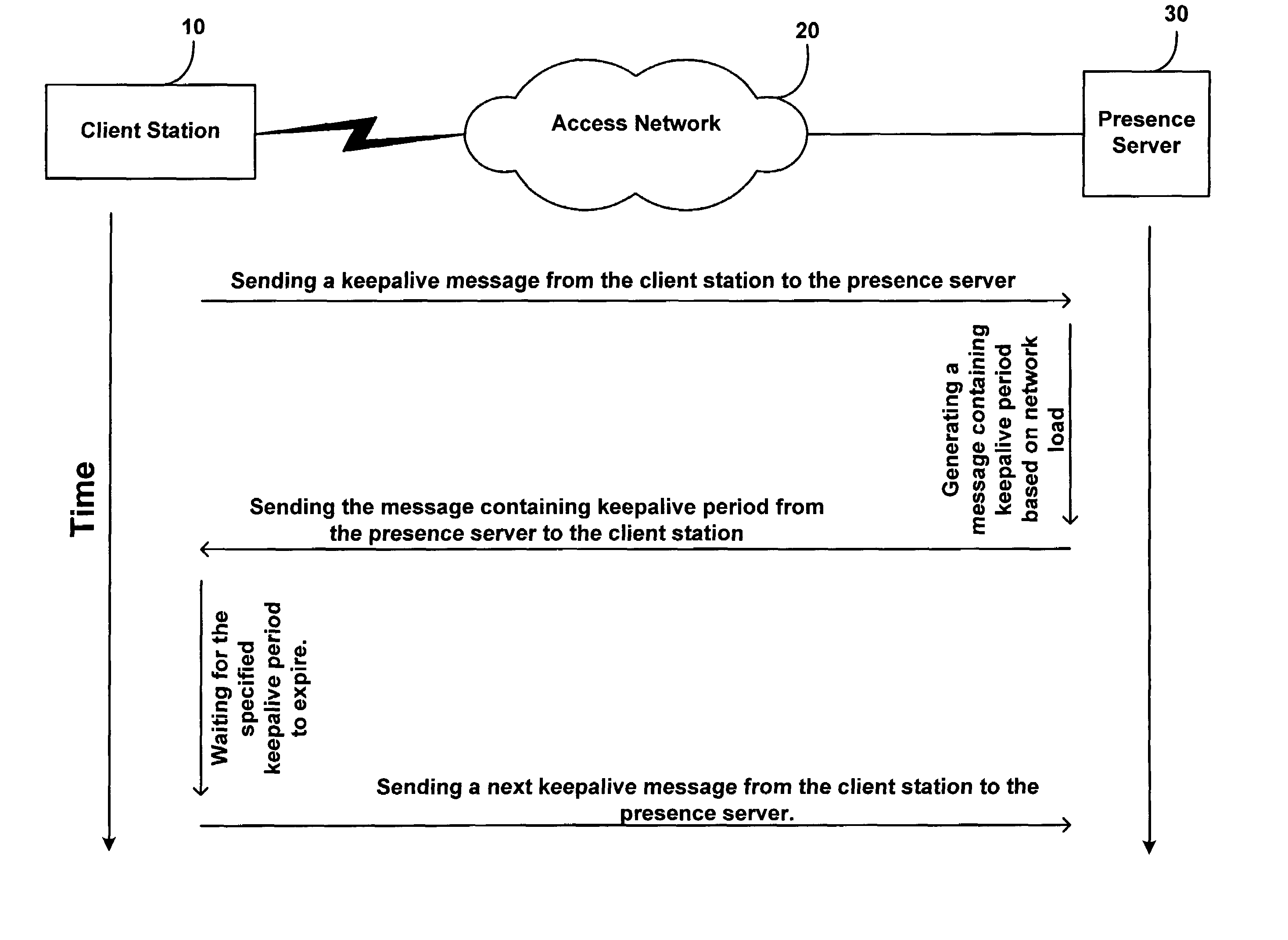
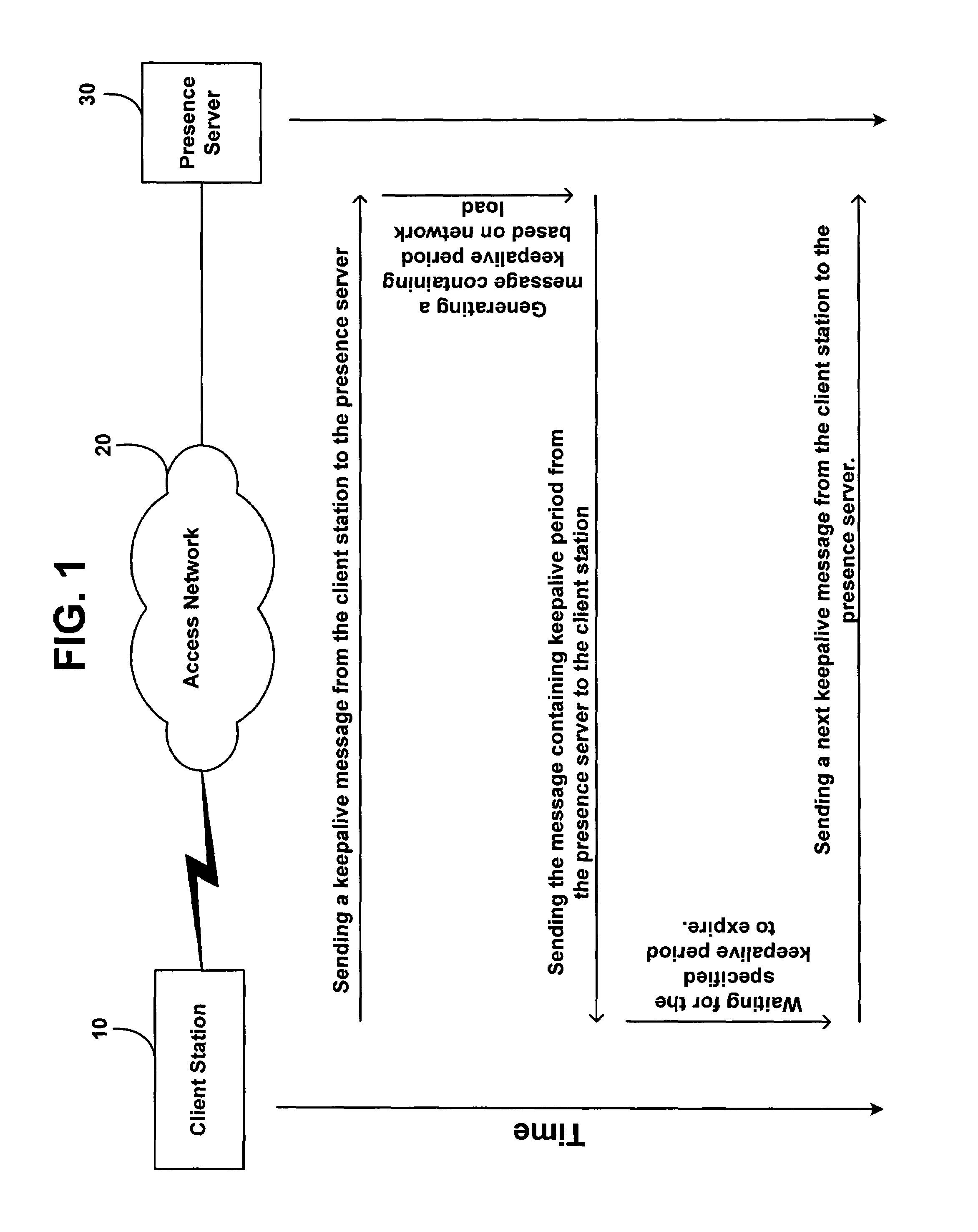
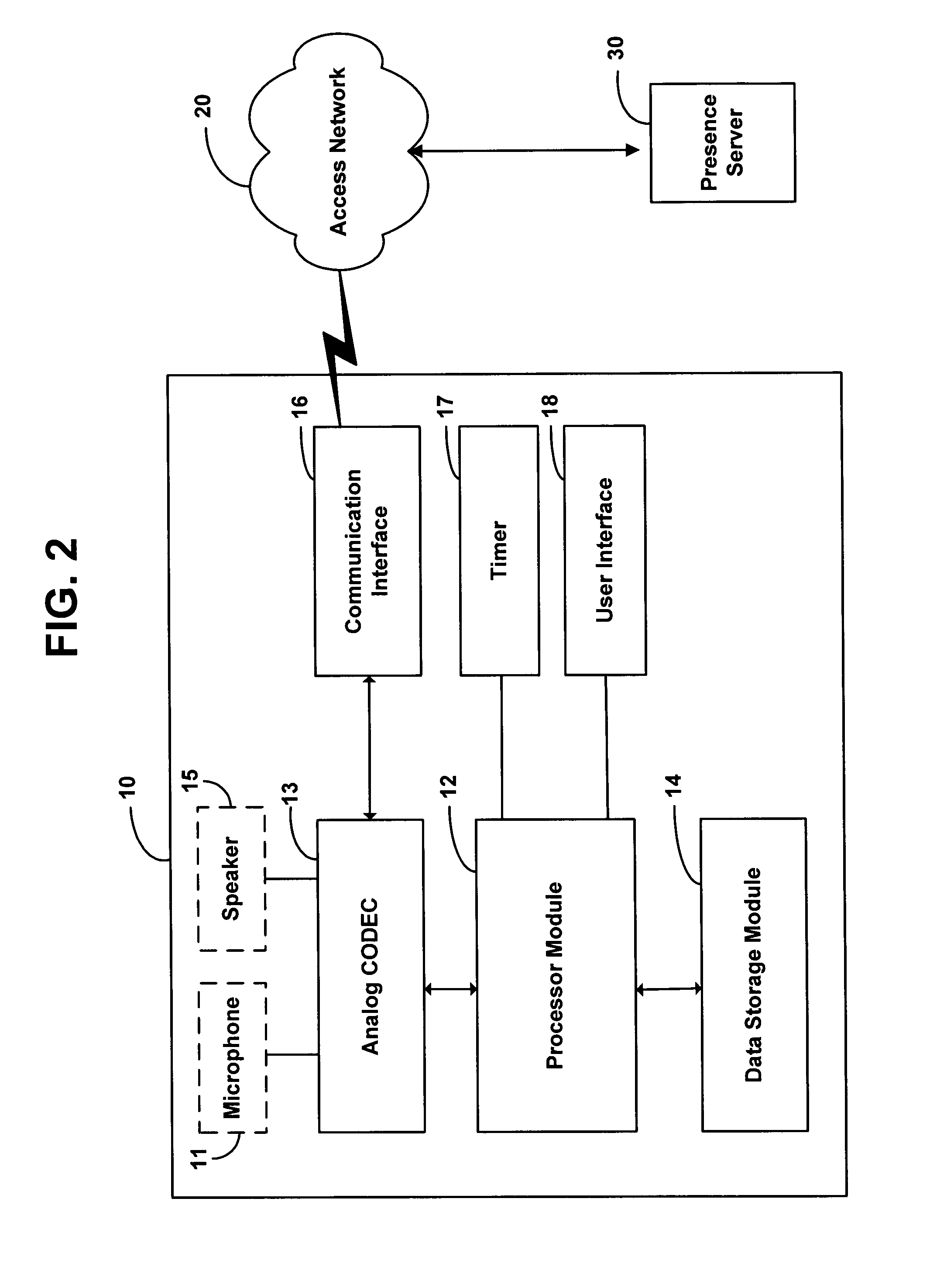
Method and system for updating network presence records at a rate dependent on network load
ActiveUS7634558B1Available bandwidthSimple methodMultiple digital computer combinationsTransmissionActive messageRate dependent
A method is disclosed for determining how often a client station in a network should send keepalive messages. Based on a measure of network load, a presence server determines a keepalive period, which is a time interval in which a client station needs to send a keepalive message, and the presence server reports this keepalive period to the client station. The client station responsively sends a keepalive message to the presence server within the determined keepalive period.
Owner:SPRINT SPECTRUM LLC
Deposition apparatus for temperature sensitive materials
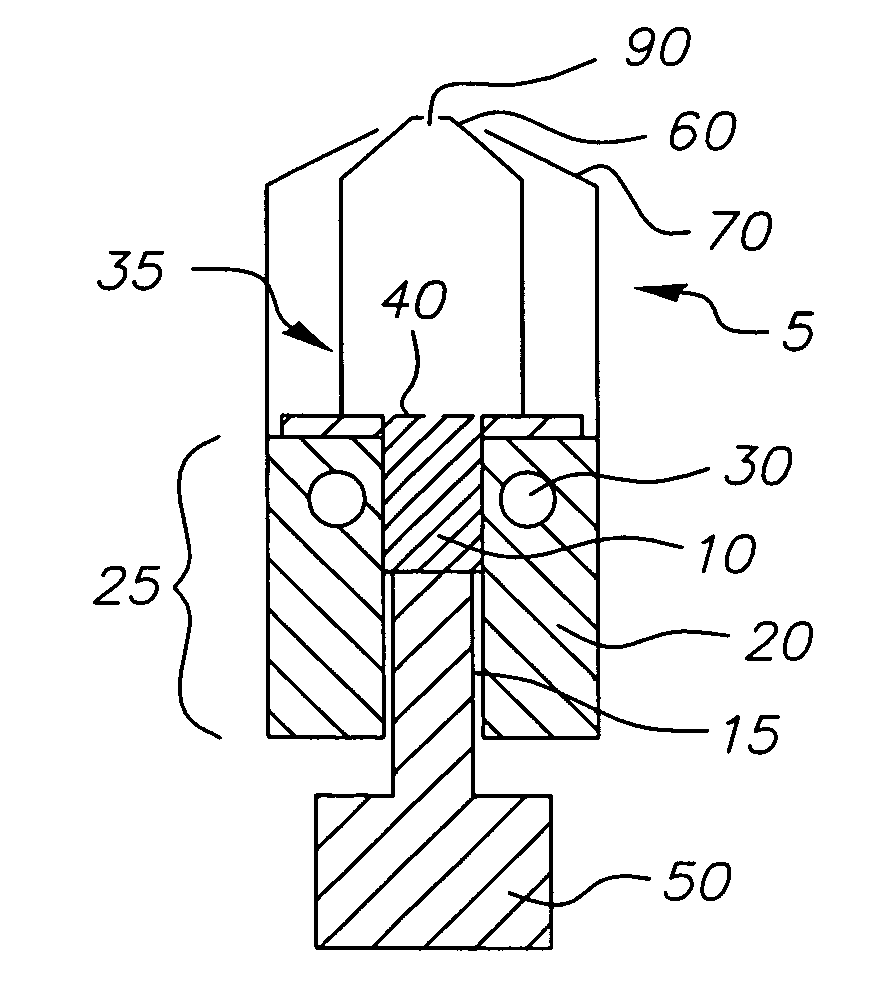
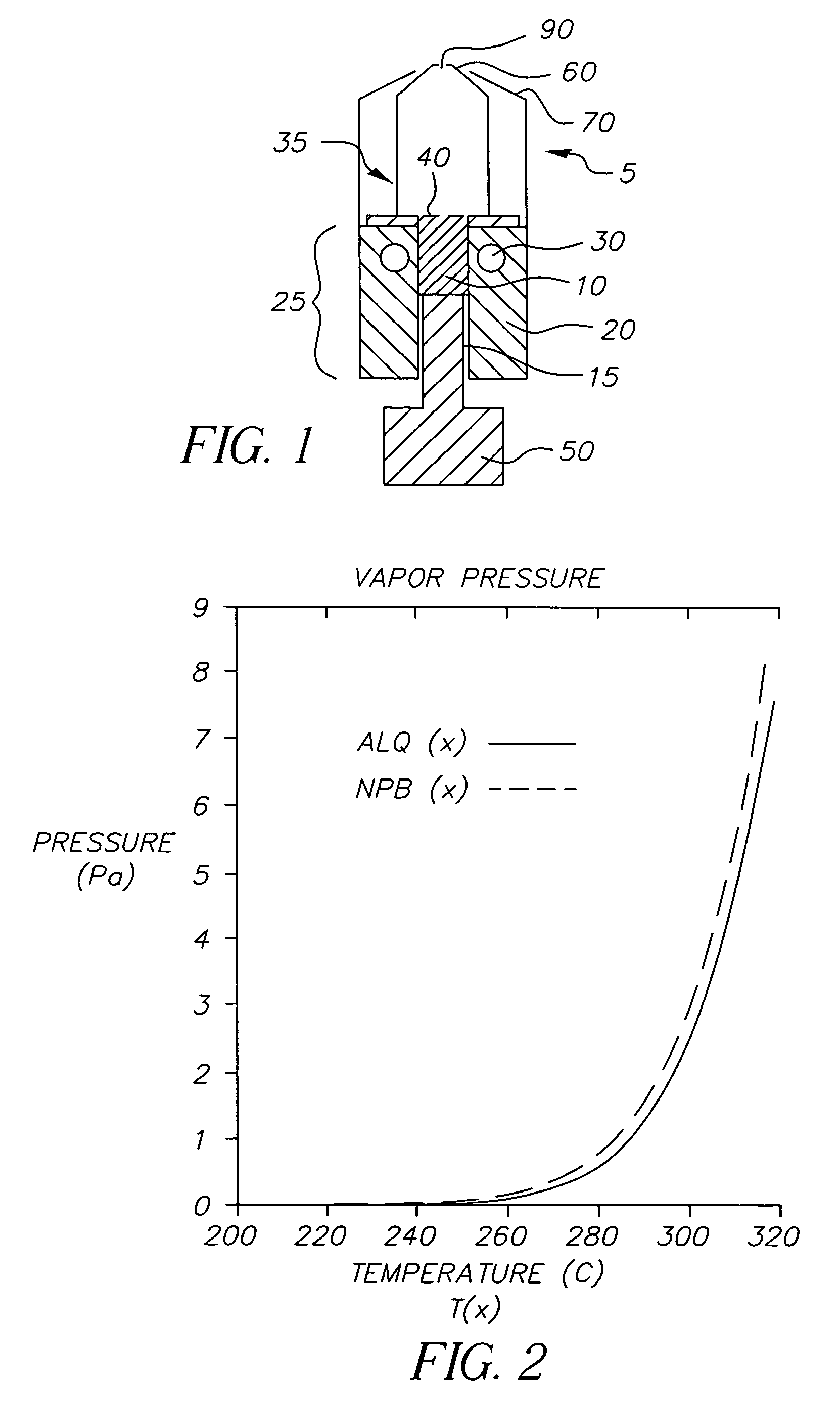
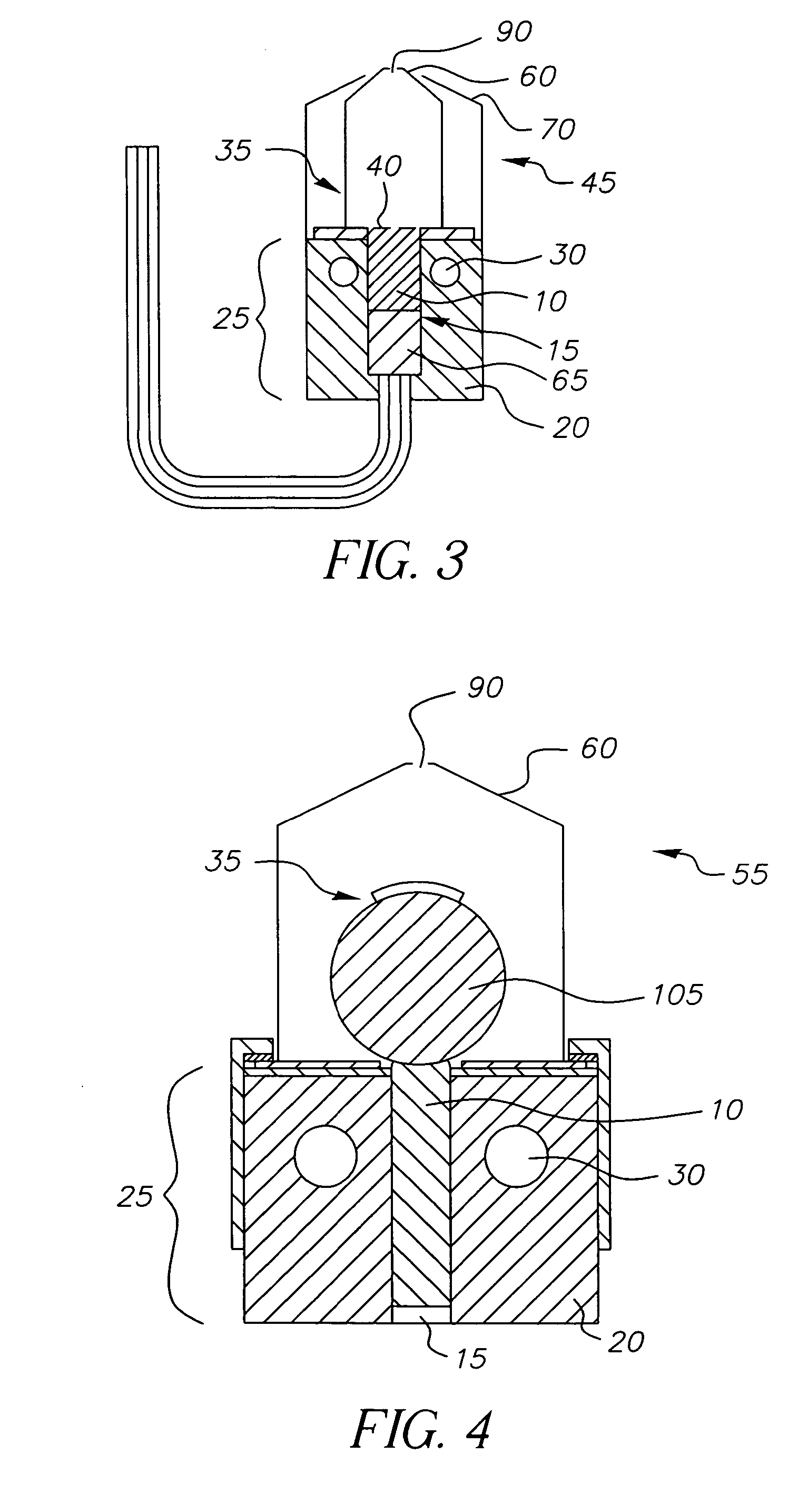
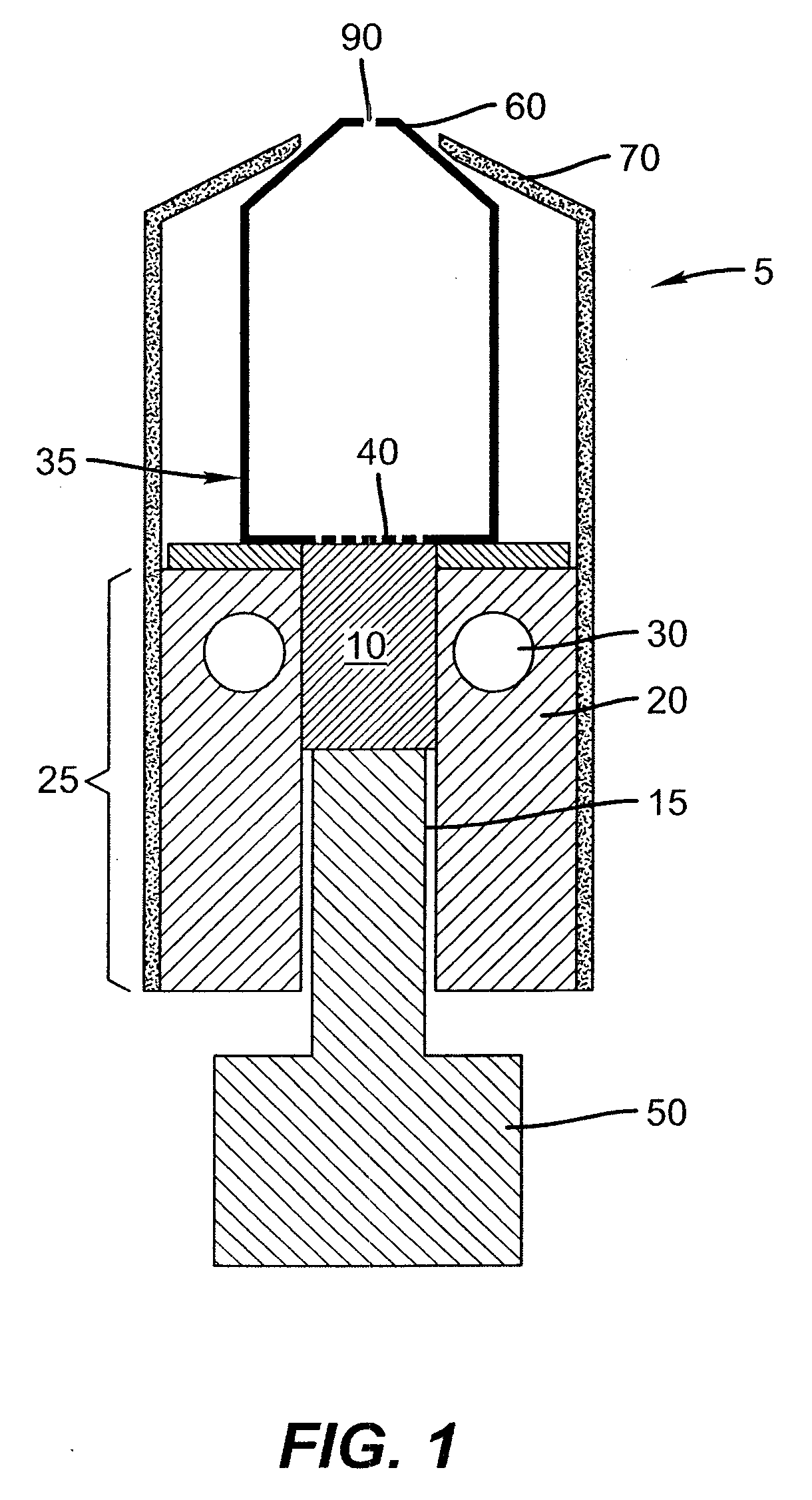
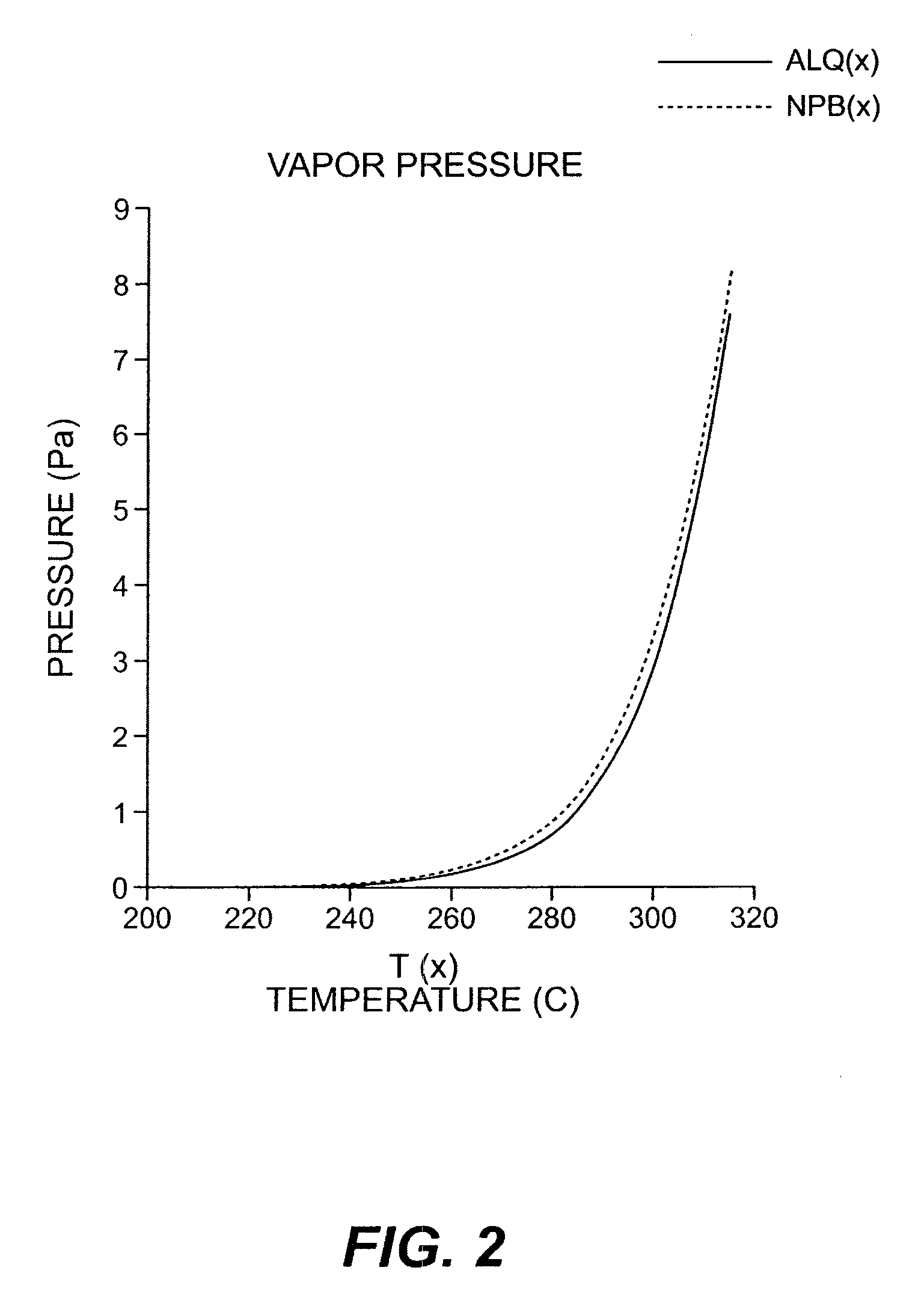
InactiveUS20050244580A1Low costImproved deposition rate controlElectroluminescent light sourcesSolid-state devicesVaporizationSubstrate surface
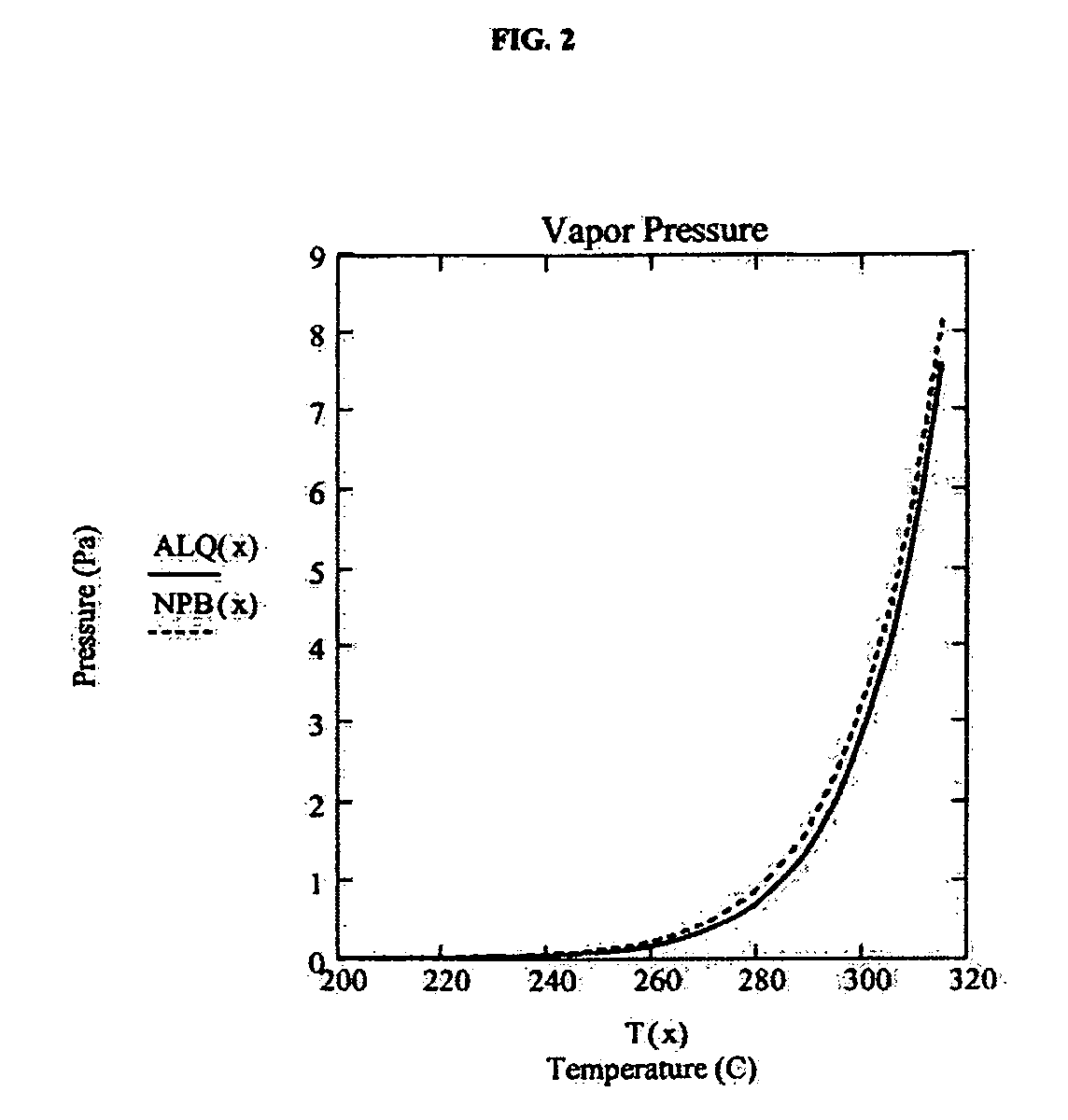
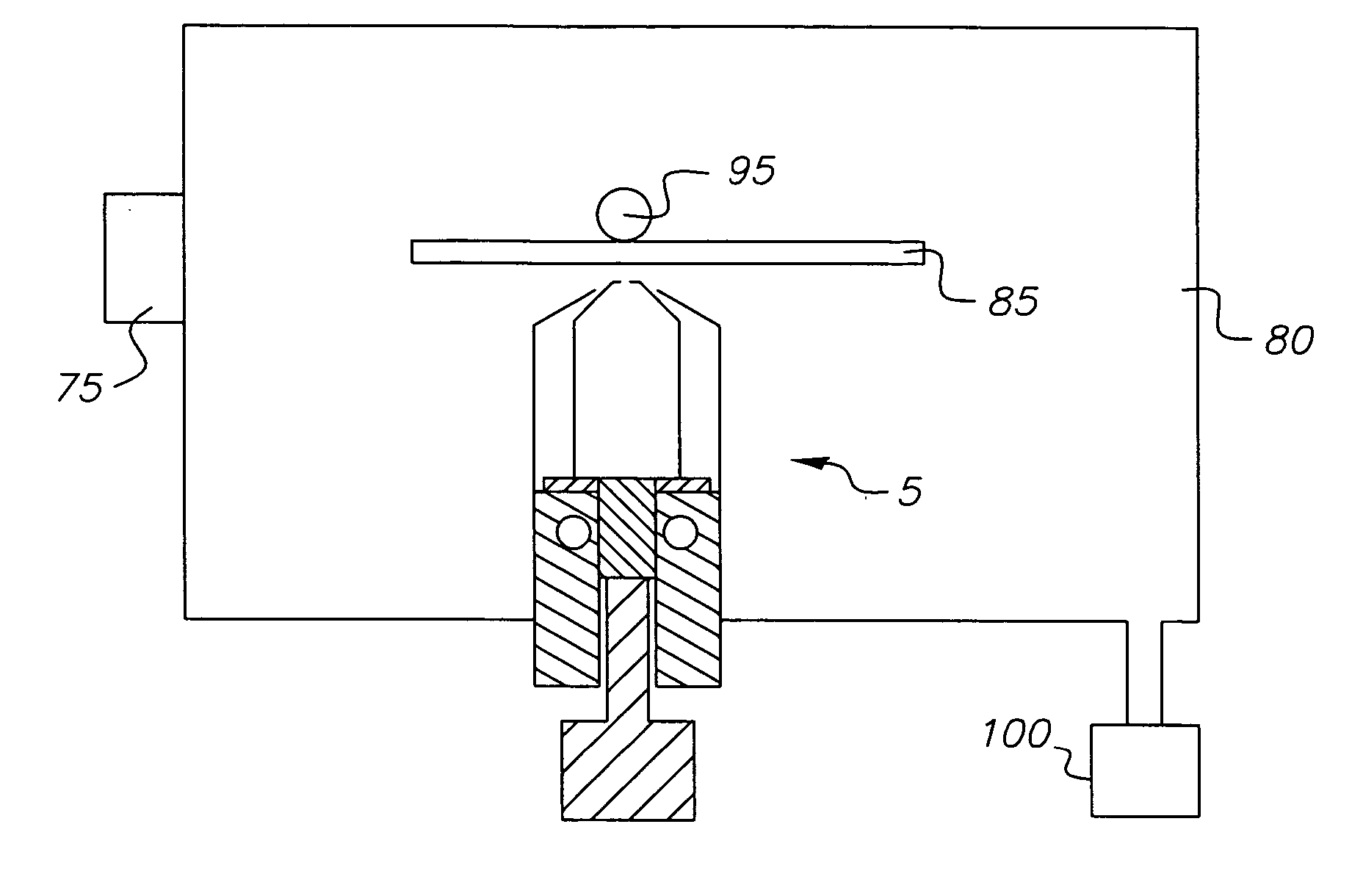
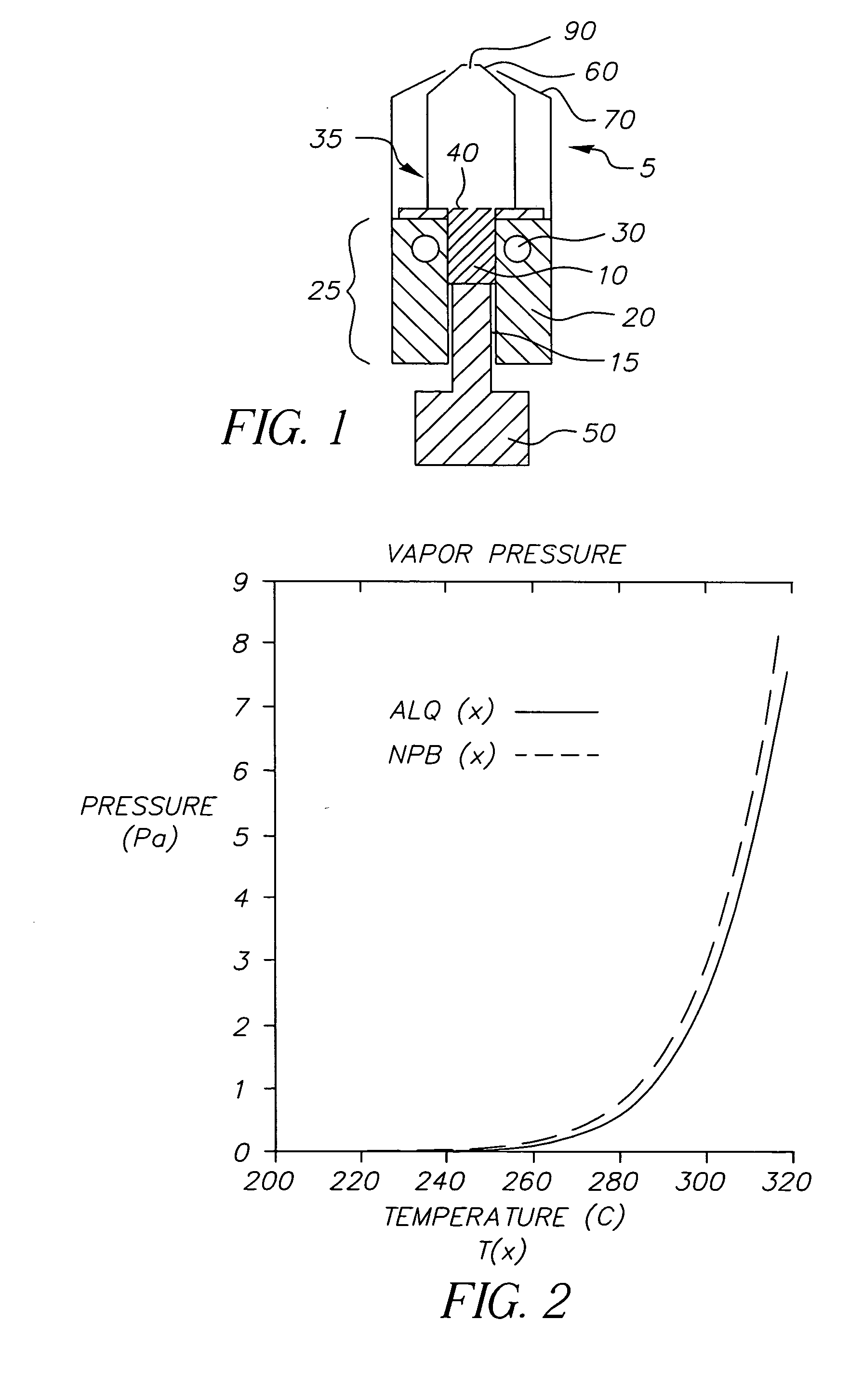
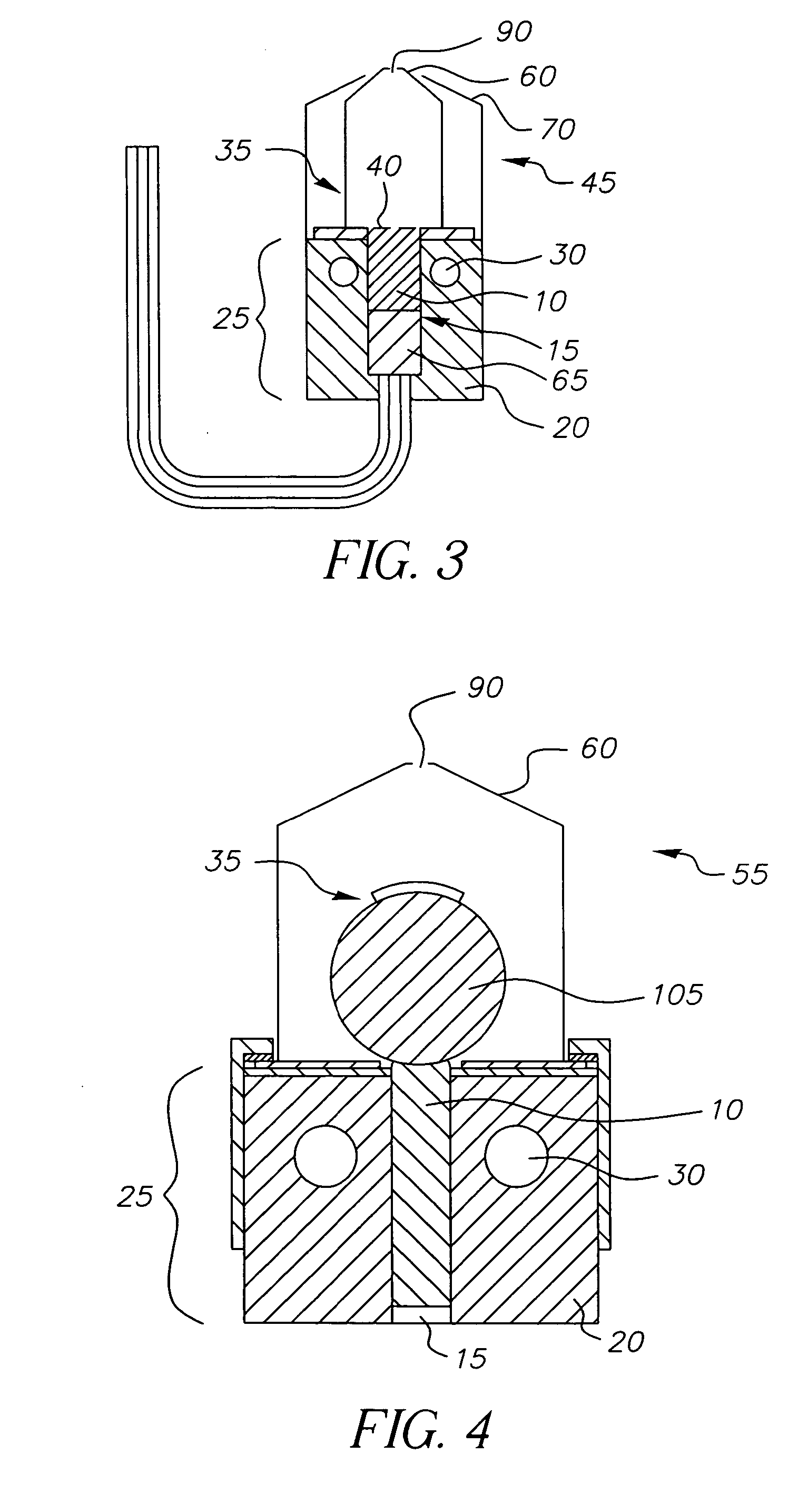
A system for the deposition of vaporized materials on a substrate is described, comprising at least first and second orientation-independent apparatuses for directing vaporized organic materials onto a substrate surface to form first and second films, each of the first and second orientation-independent apparatuses being arranged in a different relative orientation and comprising: a chamber containing a quantity of material; a permeable member at one end of the chamber with a heating element for vaporizing the material; and a piston at the other end of the chamber for continuously feeding the material toward the permeable member as it is vaporized, whereby organic material vaporizes at a desired rate-dependent vaporization temperature at the one end of the chamber. A plurality of thin films may be deposited on a substrate using deposition apparatus in a variety of orientations. Such a design provides reduced costs and improved deposition rate control.
Owner:EASTMAN KODAK CO
Device and method for vaporizing temperature sensitive materials
ActiveUS20050186340A1Steady vaporization rateReduce riskDischarge tube luminescnet screensElectroluminescent light sourcesMetallurgyVaporization
A method for vaporizing organic materials onto a substrate surface to form a film including providing a quantity of organic material into a vaporization apparatus and actively maintaining the organic material in a first heating region in the vaporization apparatus to be below the vaporization temperature. The method also includes heating a second heating region of the vaporization apparatus above the vaporization temperature of the organic material and metering, at a controlled rate, organic material from the first heating region into the second heating region so that a thin cross section of the organic material is heated at a desired rate-dependent vaporization temperature, whereby organic material vaporizes and forms a film on the substrate surface.
Owner:GLOBAL OLED TECH
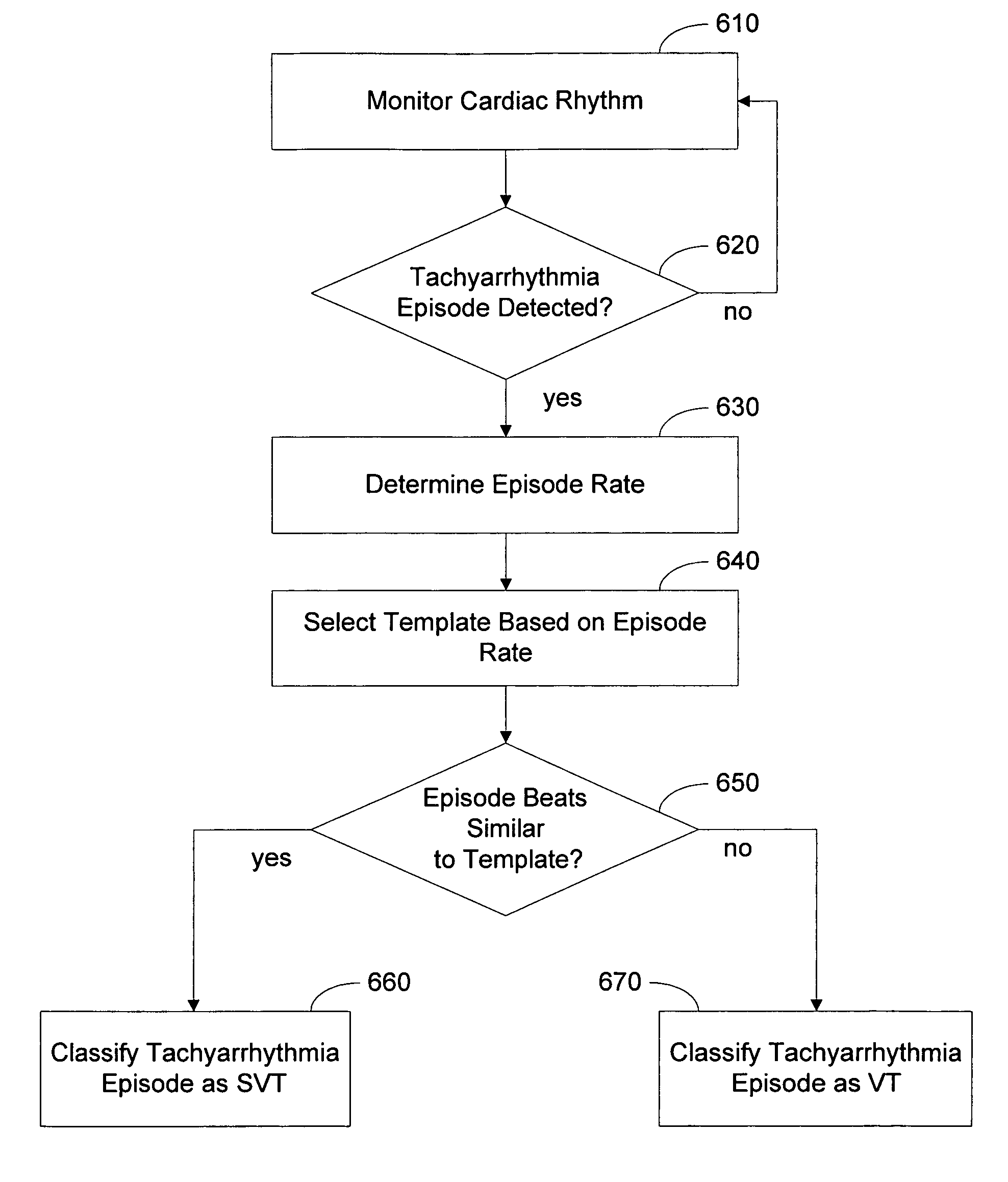
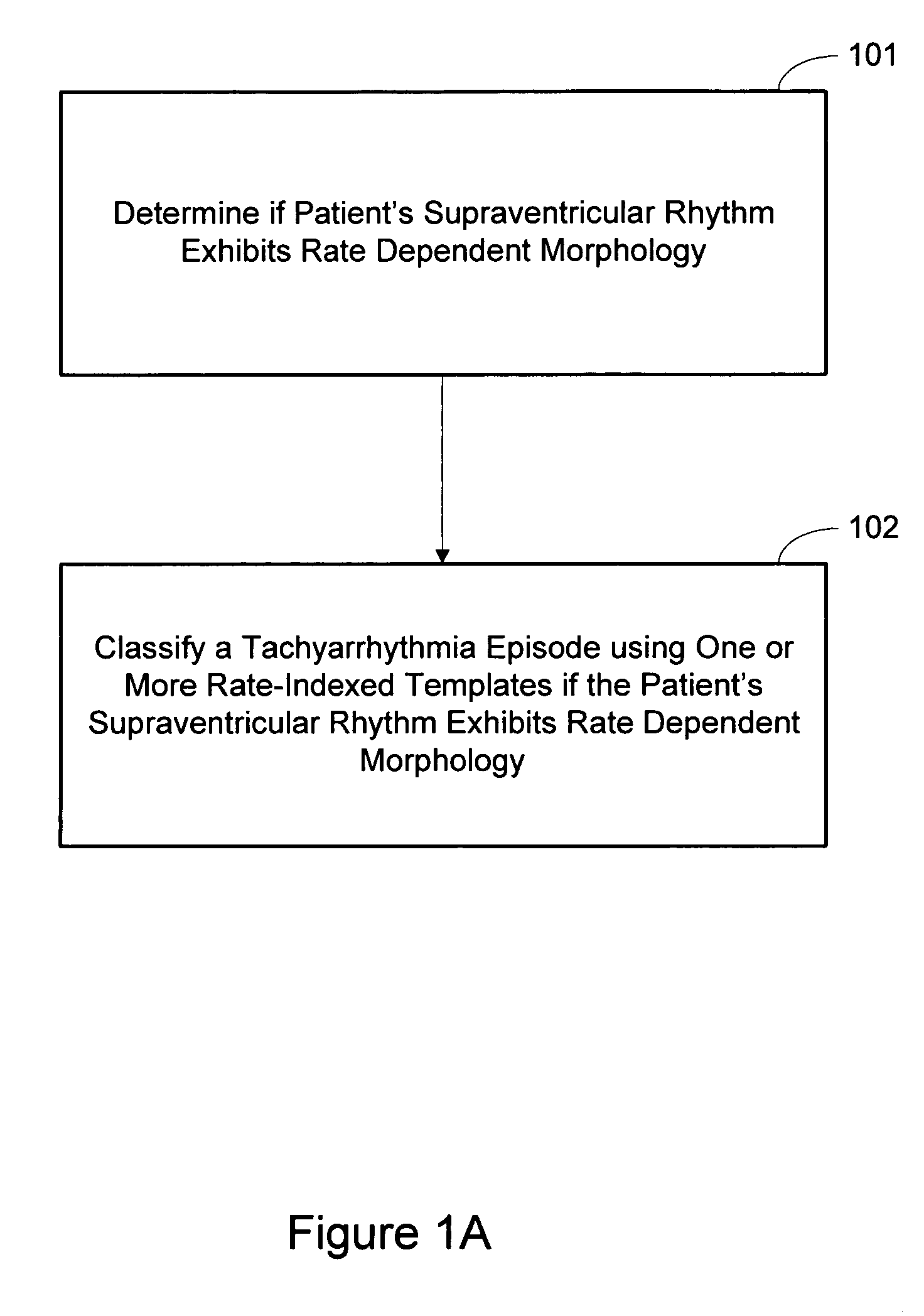
Arrhythmia discrimination based on determination of rate dependency
InactiveUS7653431B2ElectrocardiographyMedical automated diagnosisVentricular TachyarrhythmiasRate dependent
Cardiac systems and methods provide for discriminating between supraventricular tachyarrhythmia and ventricular tachyarrhythmia based on a determination that the patient's supraventricular rhythm exhibits rate dependency. One approach involves determining if a patient's supraventricular rhythm exhibits rate dependent morphology. If the patient's supraventricular rhythm is determined to exhibit rate dependent morphology, an implantable device classifies a detected tachyarrhythmia episode based on one or more templates selected from a plurality of rate-indexed templates stored in the device. Determining if the supraventricular rhythm exhibits rate dependent morphology may also include determining one or more rates at which the rate dependent morphology occurs.
Owner:CARDIAC PACEMAKERS INC
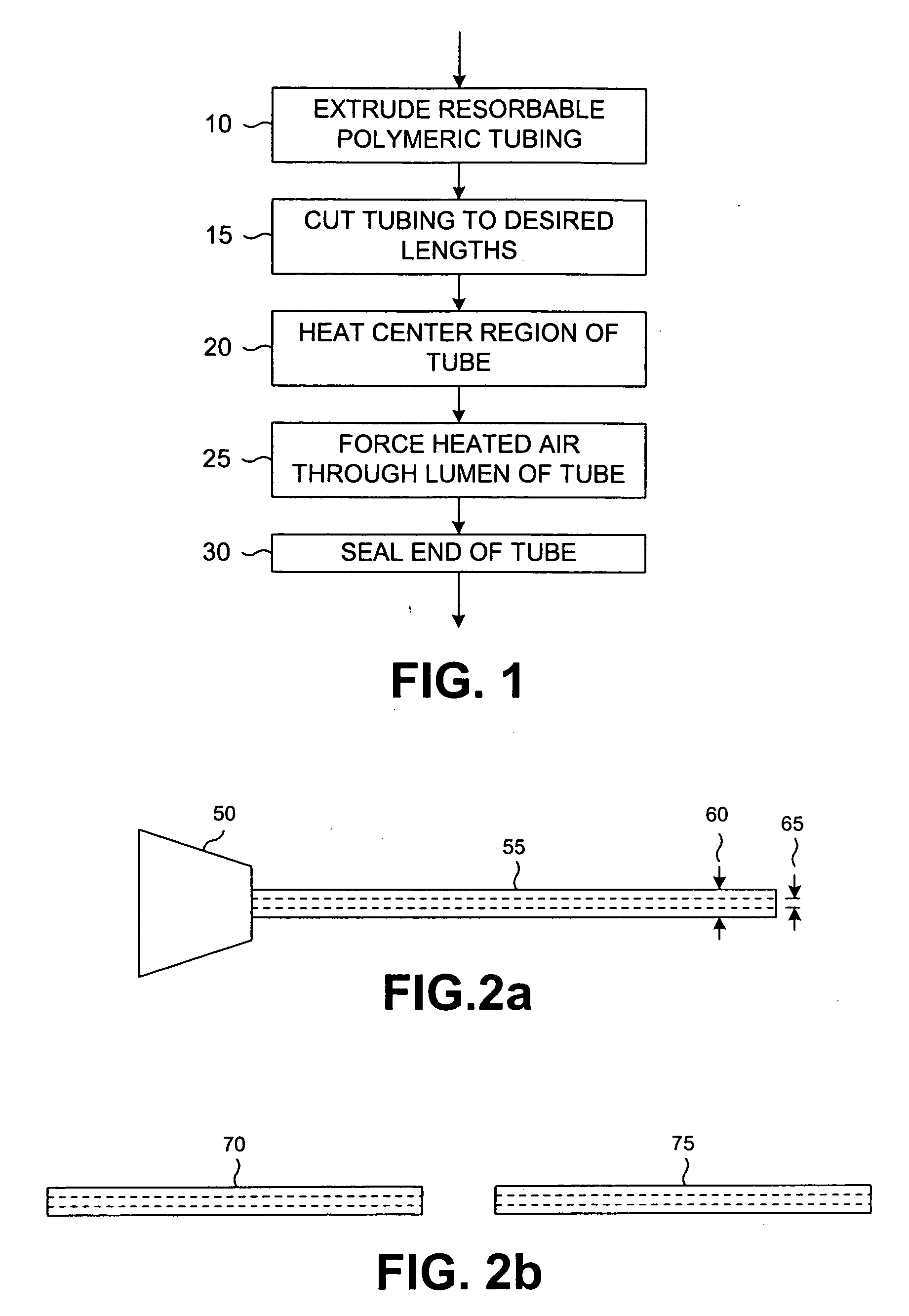
Resorbable hollow devices for implantation and delivery of therapeutic agents
InactiveUS20060182780A1Prevent intrusionAvoiding and attenuating problemStentsBalloon catheterCavitationMedicine
A method of manufacturing a resorbable balloon designed to contain bone cement for vertebroplasty or kyphoplasty applications is described. The resorbable balloon can be inserted into a vertebral body following vertebral cavitation and filled with bone cement. The balloon remains in place in the vertebral body and resorbs over time. Methods and apparatus are also described for delivering therapeutic agents using collapsible, resorbable balloons. The balloons may be nested and filled with various therapeutic agents that are released over time at rates dependent upon structures and degradation rates of the balloons. Furthermore, the function of the hollow devices can encompass both encapsulation and therapeutic substance delivery roles simultaneously.
Owner:VACCARO ALEXANDER R
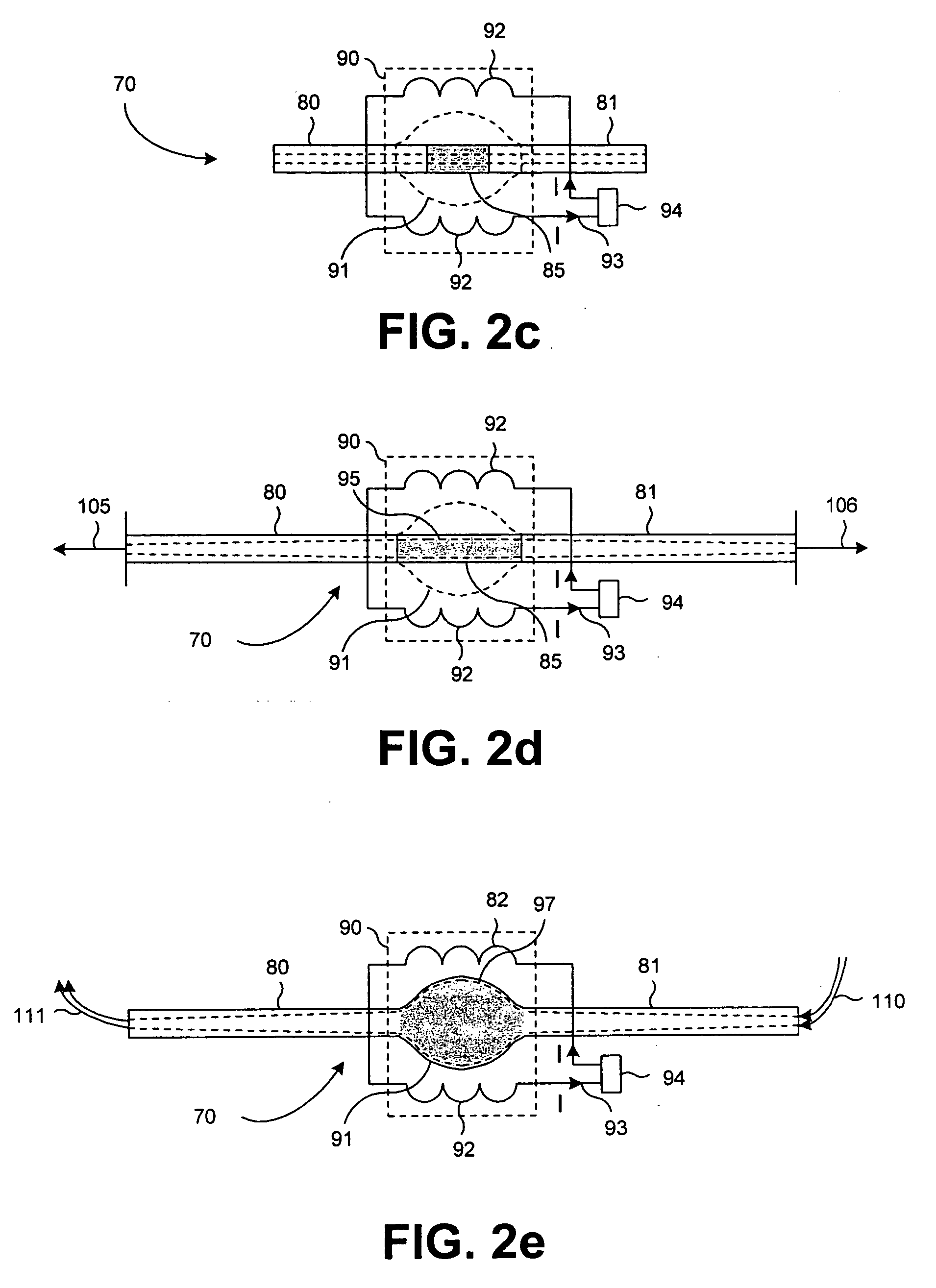
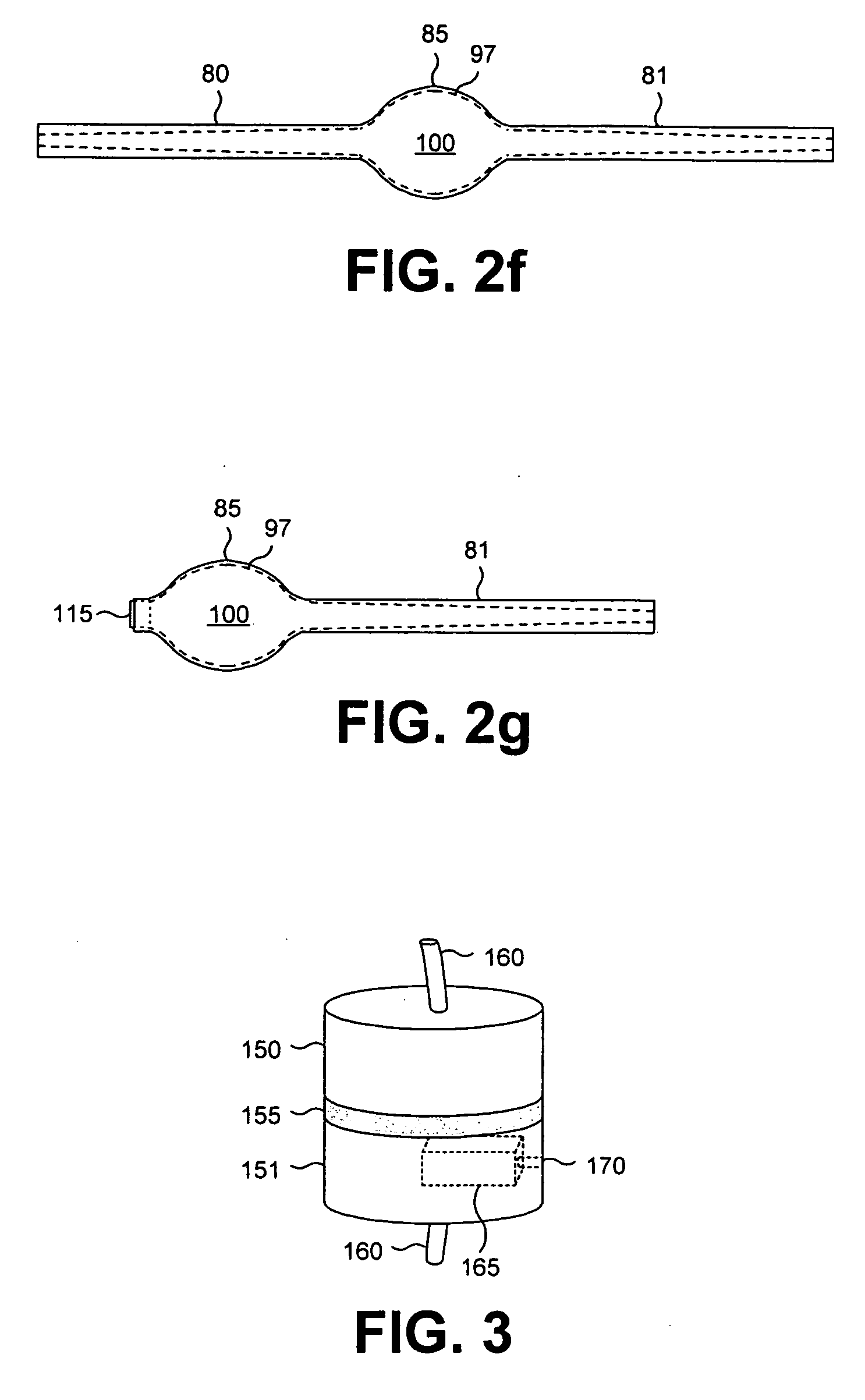
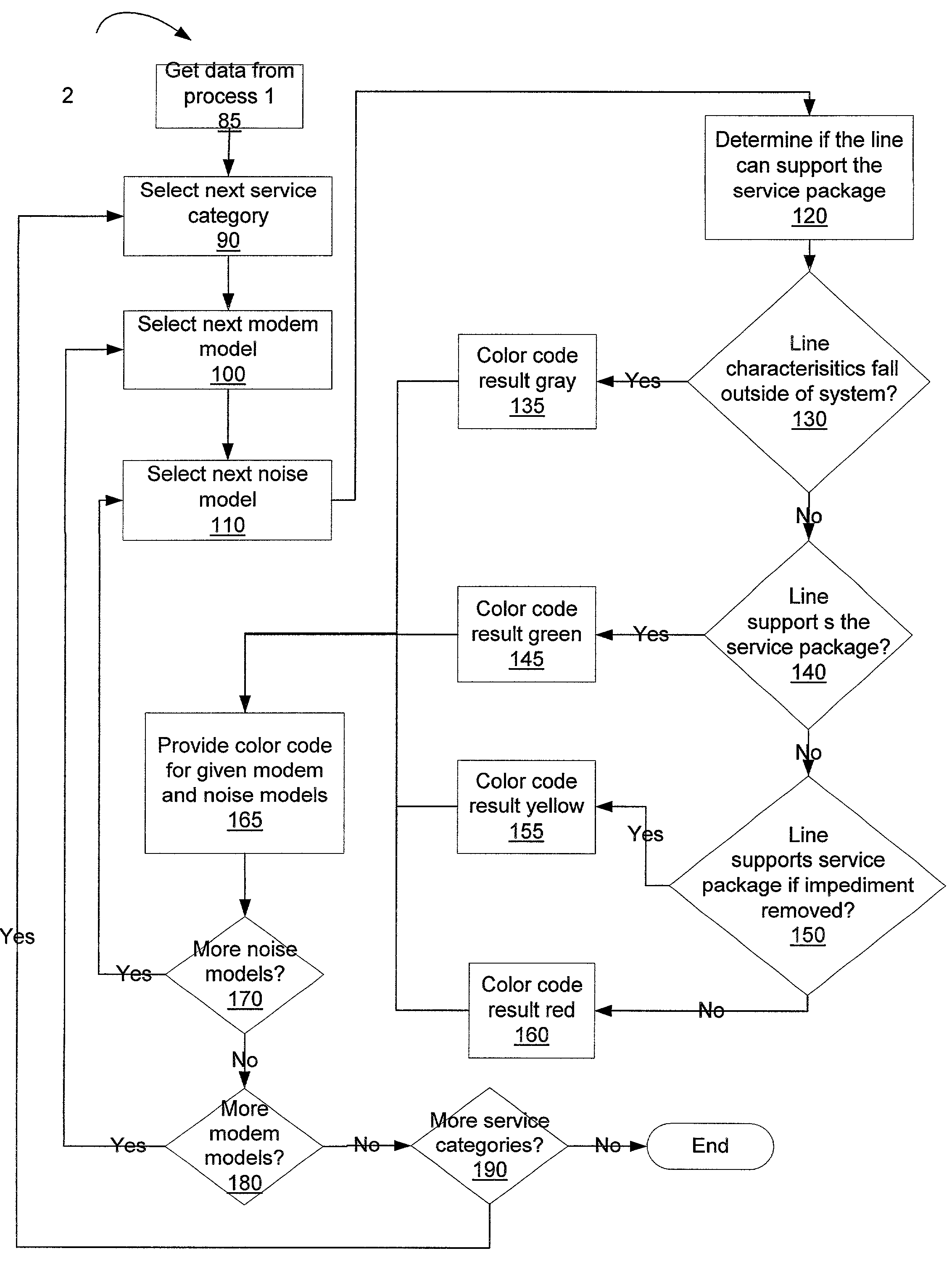
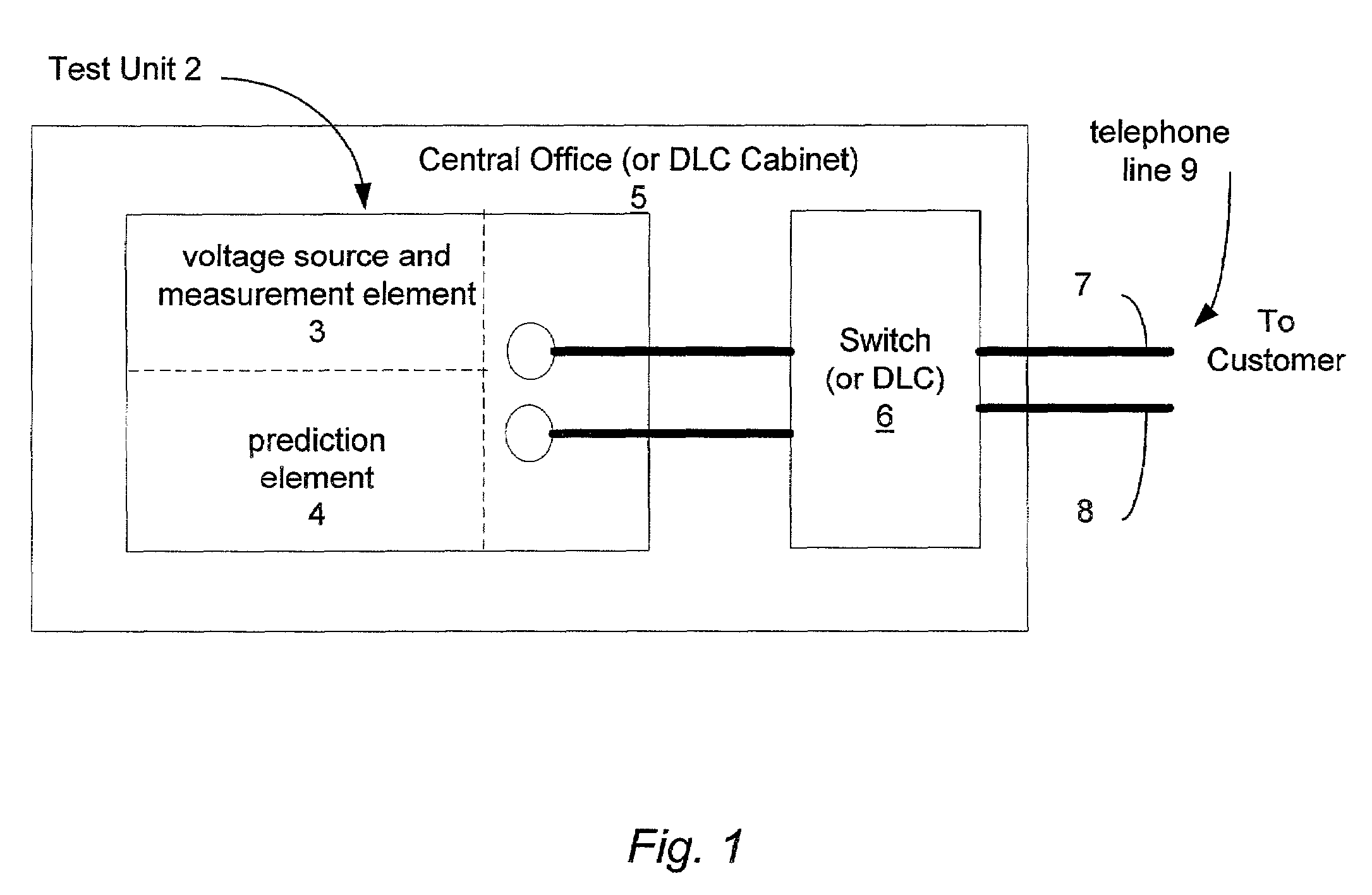
Binning of results from loop qualification tests
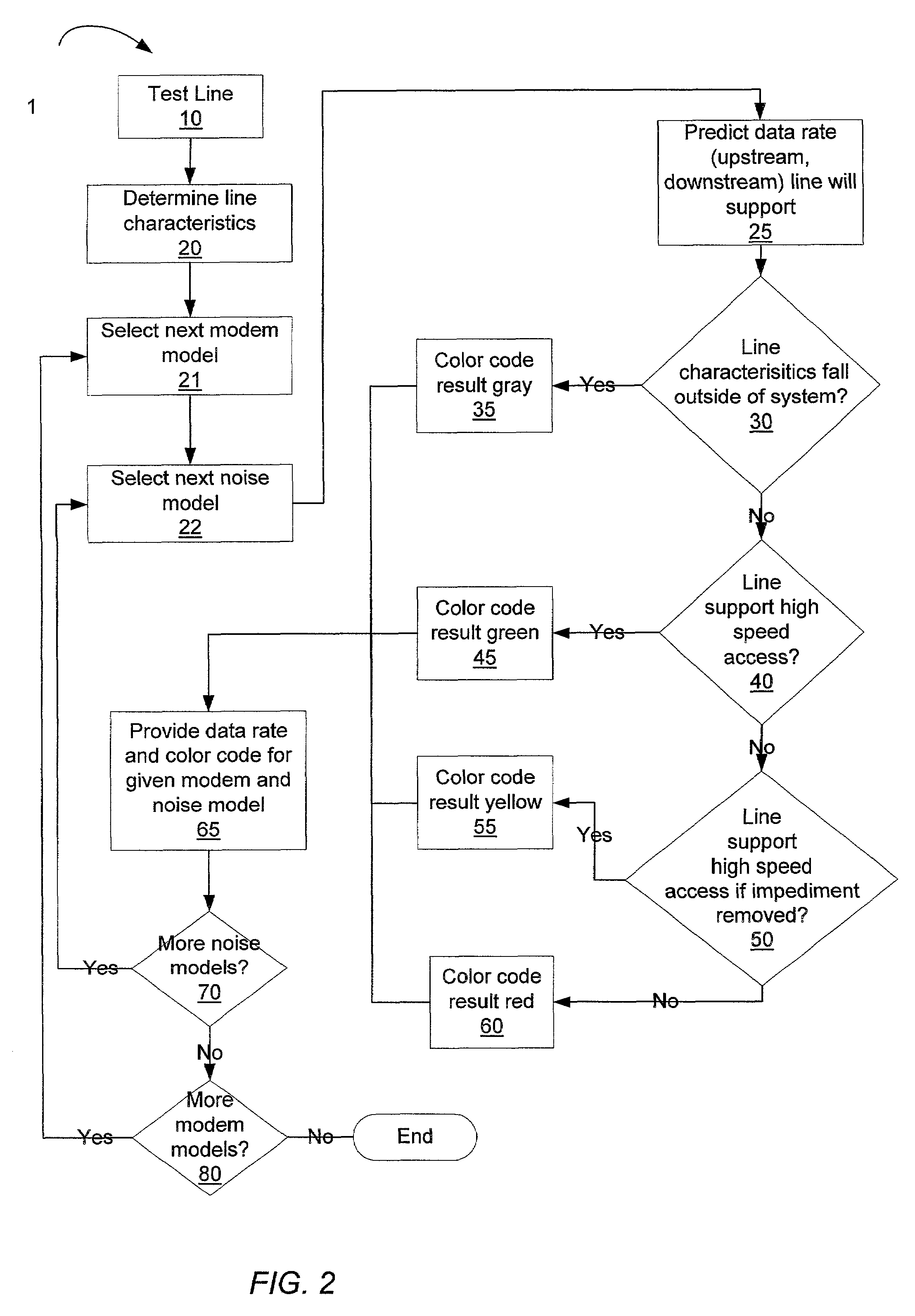
InactiveUS6985444B1Easily discernable statusError preventionFrequency-division multiplex detailsData rateSoftware design
The present invention provides a method and apparatus for performing line qualification tests, and binning the results of such testing. The lines are tested to determine or estimate various characteristics of the line. Physical characteristics of the line may be estimated (e.g. line length, line gauge, insertion loss). The presence of devices on the line such as load coils, bridged taps, terminations and the like may also be determined. A prediction of the data rate the loop can support is made from the measured and estimated line conditions. The results are binned according to certain criteria and to provide an easily discernable status of the line. The binning can be performed by a computer using software designed specifically for this purpose. The binned results may include a first category indicating the line cannot support a certain level of high speed access. The binned results may also include a second category indicating the line can support a certain level of high speed access. The results may also include a third category indicating the line cannot currently support a certain level of high speed access but would be able to upon removal of an impediment. A fourth category indicating the characteristics of the selected line fall outside the area of coverage of the test system may also be included. Each category may be assigned a respective color in order to make the status of the line easily discernable. The testing and binning may be performed for a variety of different high speed access levels. Customers can be charged different rates dependent upon the level of service made available to them.
Owner:TOLLGRADE COMM INC
Land seismic data acquisition method and seismic cable and cable spool vehicle therefor
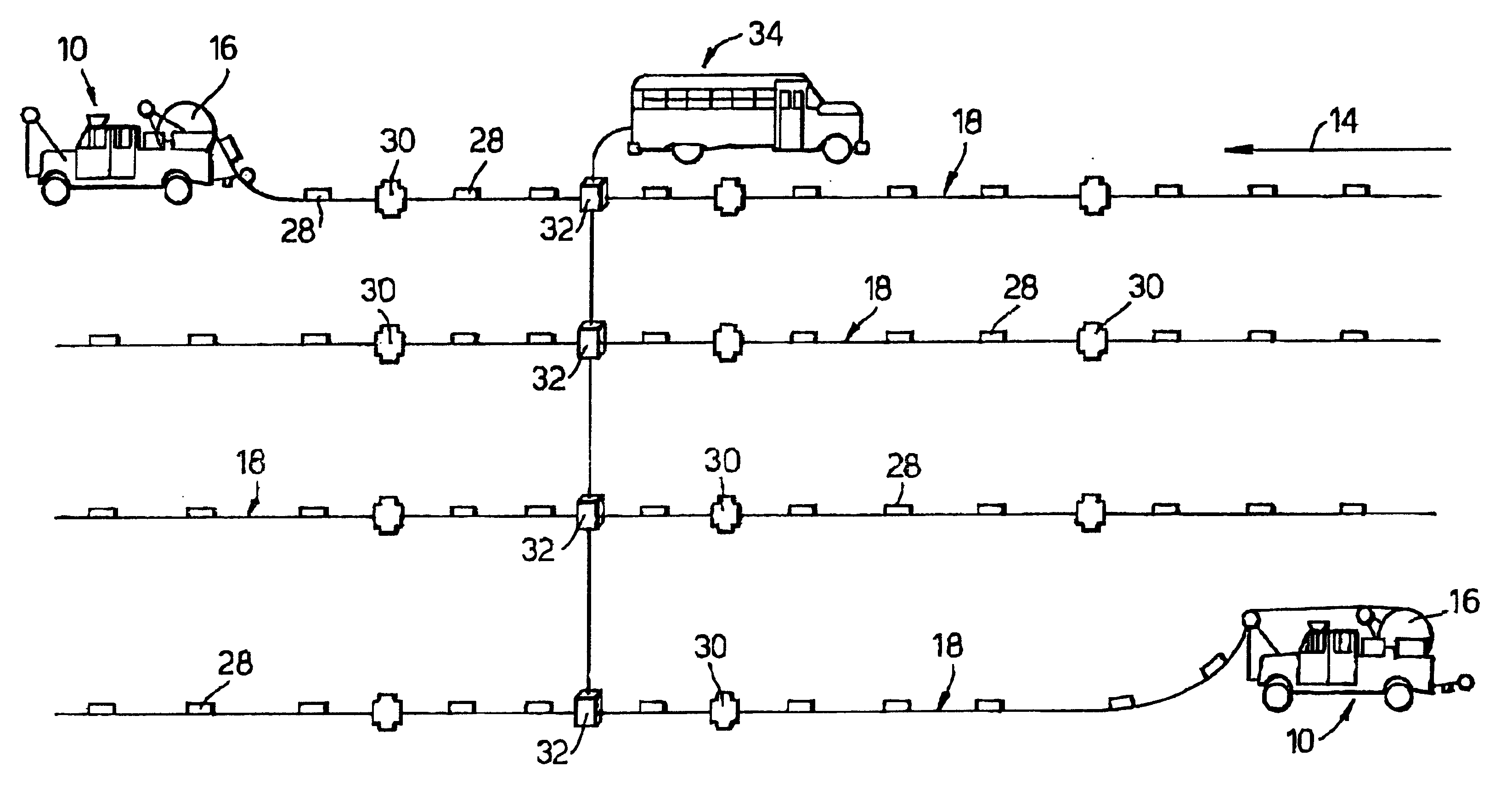
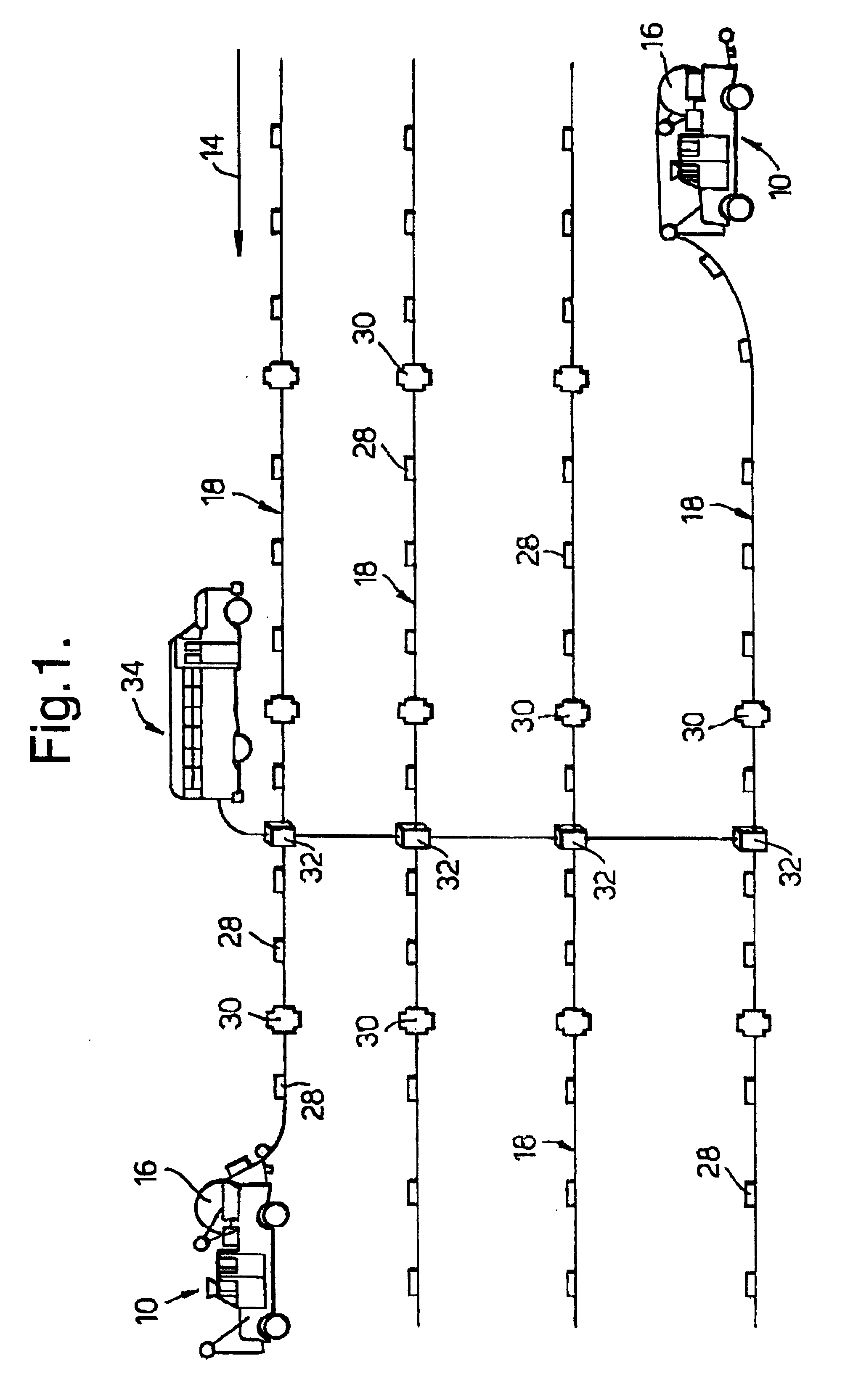
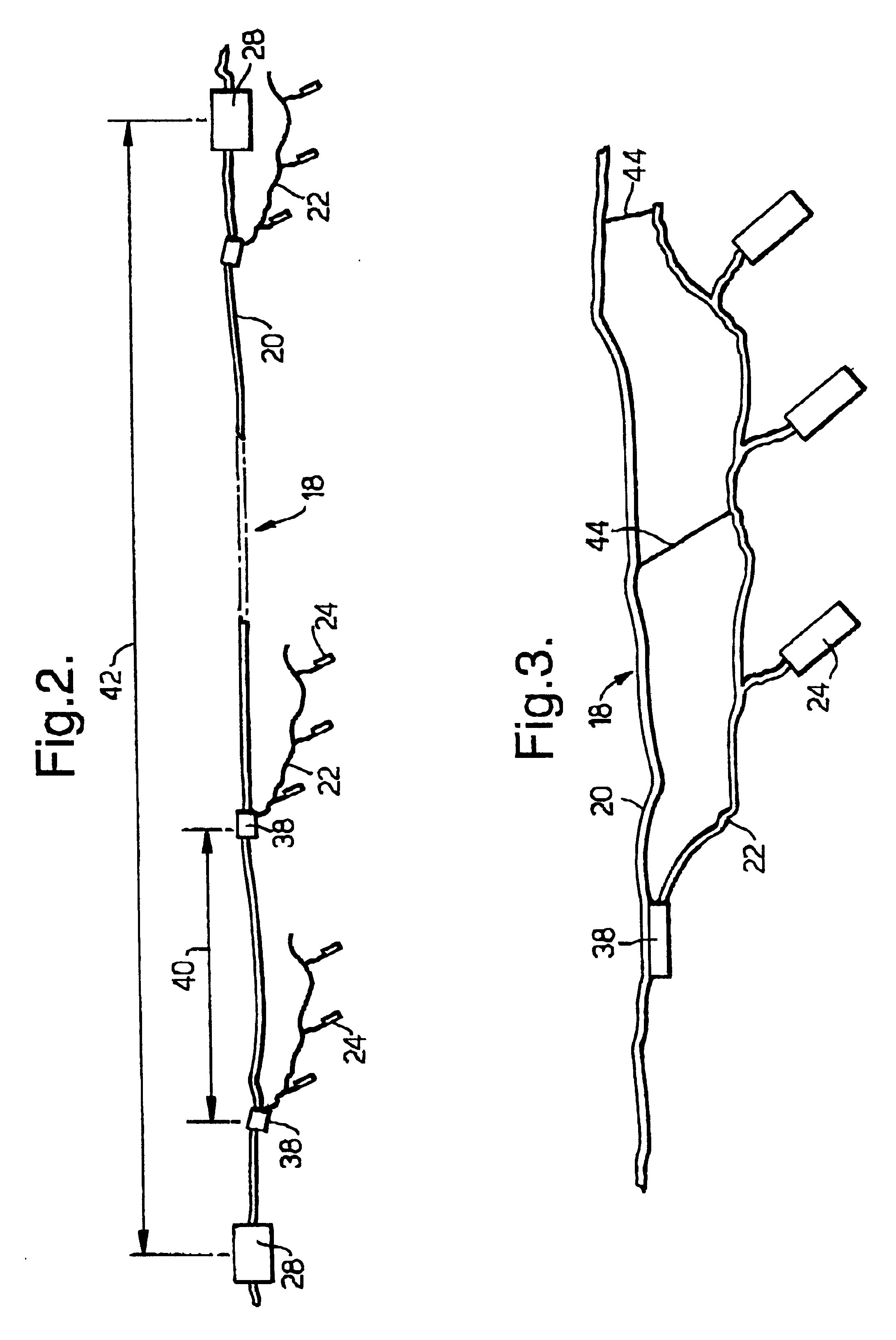
InactiveUS6260656B1Abrasion of damageAccurate couplingSonic/ultrasonic/infrasonic transmissionSeismic data acquisitionData acquisitionEngineering
A method for land seismic data acquisition is provided, together with a seismic cable and a cable spool vehicle for use in the method. In performing the method, the cable spool vehicle mechanically deploys seismic cable with attached sensors according to a desired geophysical spread and at a rate dependent upon the speed of movement of the vehicle substantially without tension in the cable. The cable spool vehicle also allows mechanical pick-up of the seismic cable together with the sensors after the seismic data acquisition.
Owner:SCHLUMBERGER TECH CORP
Device and method for vaporizing temperature sensitive materials
ActiveUS7232588B2Reduce riskStable rateDischarge tube luminescnet screensElectroluminescent light sourcesMetallurgyVaporization
A method for vaporizing organic materials onto a substrate surface to form a film including providing a quantity of organic material into a vaporization apparatus and actively maintaining the organic material in a first heating region in the vaporization apparatus to be below the vaporization temperature. The method also includes heating a second heating region of the vaporization apparatus above the vaporization temperature of the organic material and metering, at a controlled rate, organic material from the first heating region into the second heating region so that a thin cross section of the organic material is heated at a desired rate-dependent vaporization temperature, whereby organic material vaporizes and forms a film on the substrate surface.
Owner:GLOBAL OLED TECH
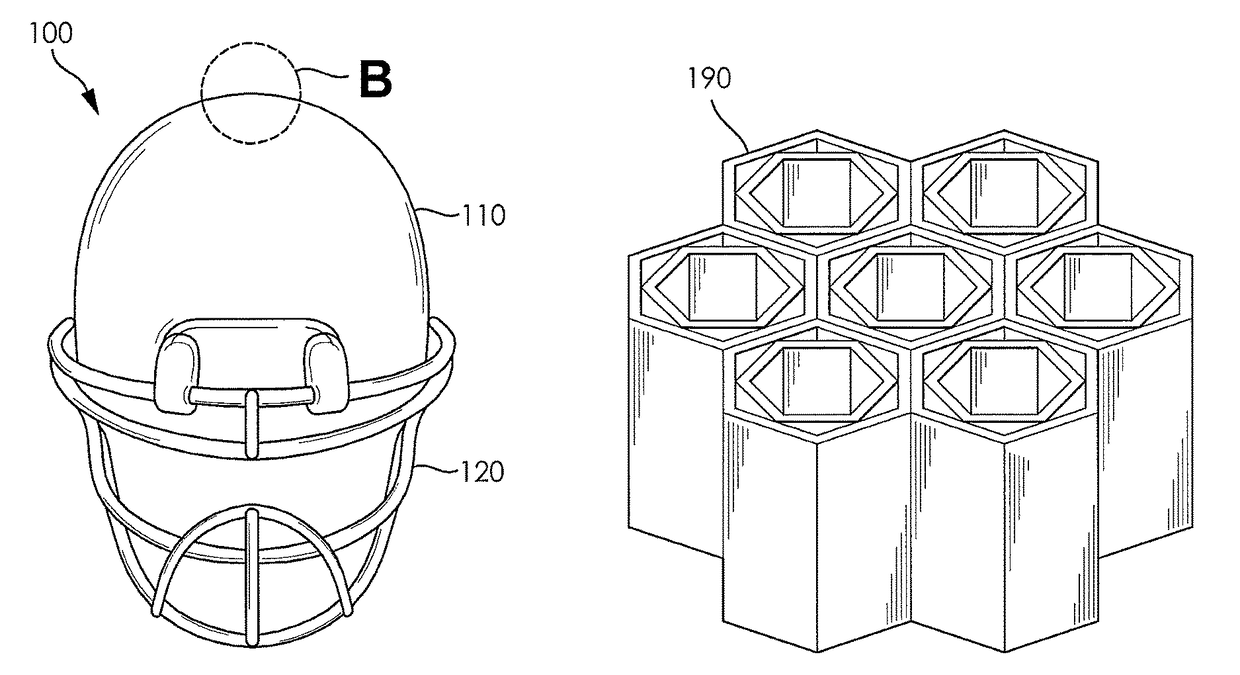
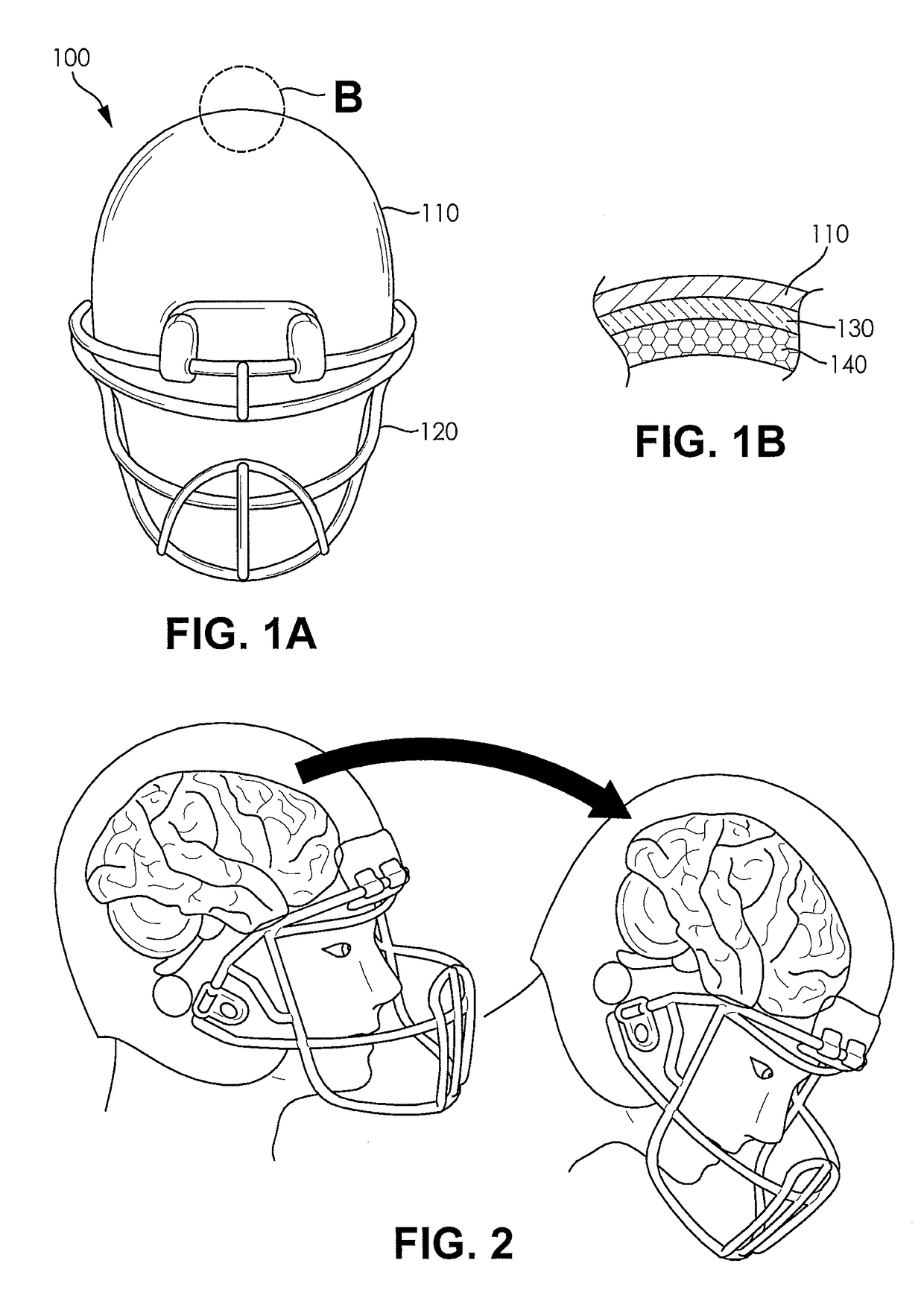
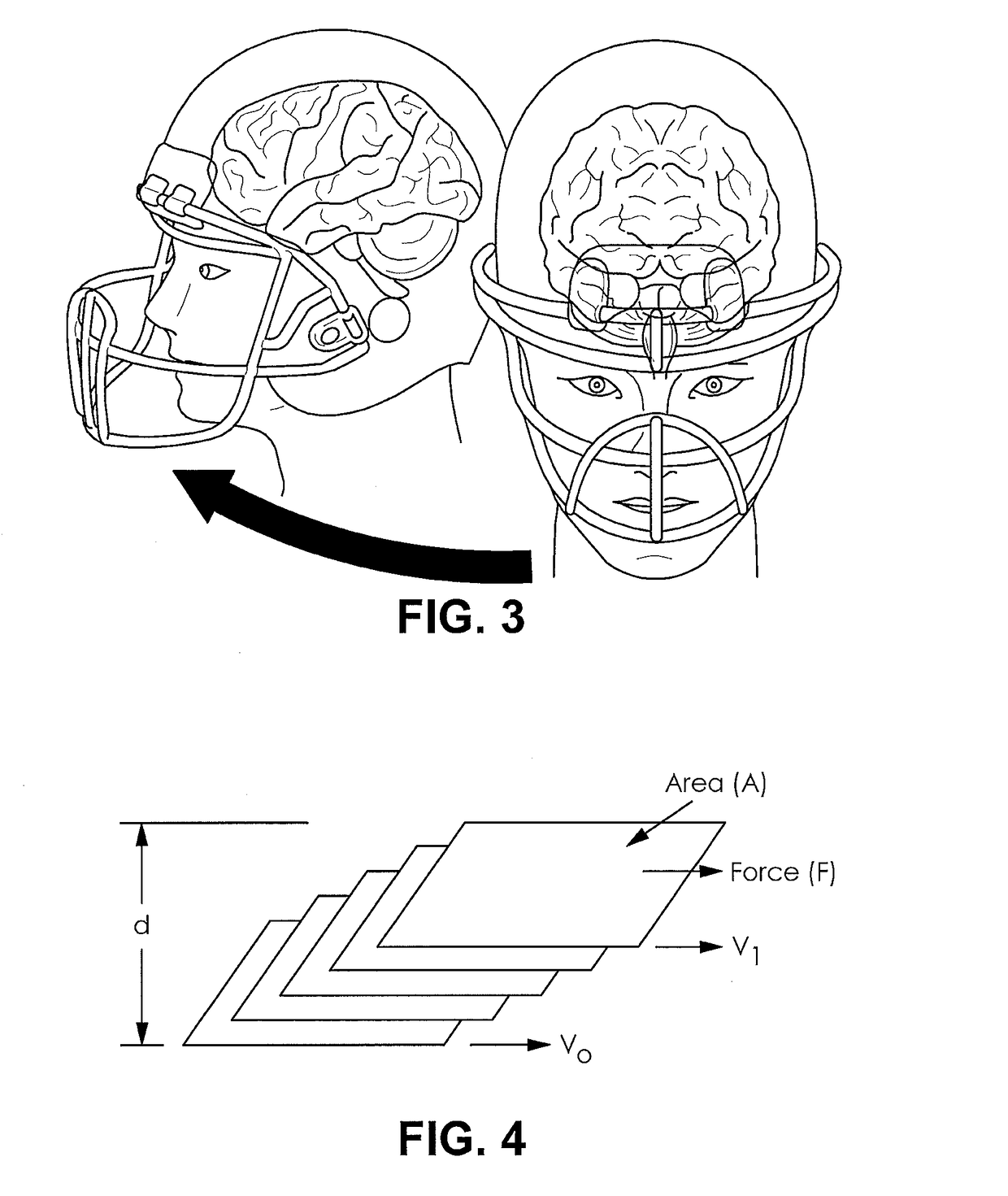
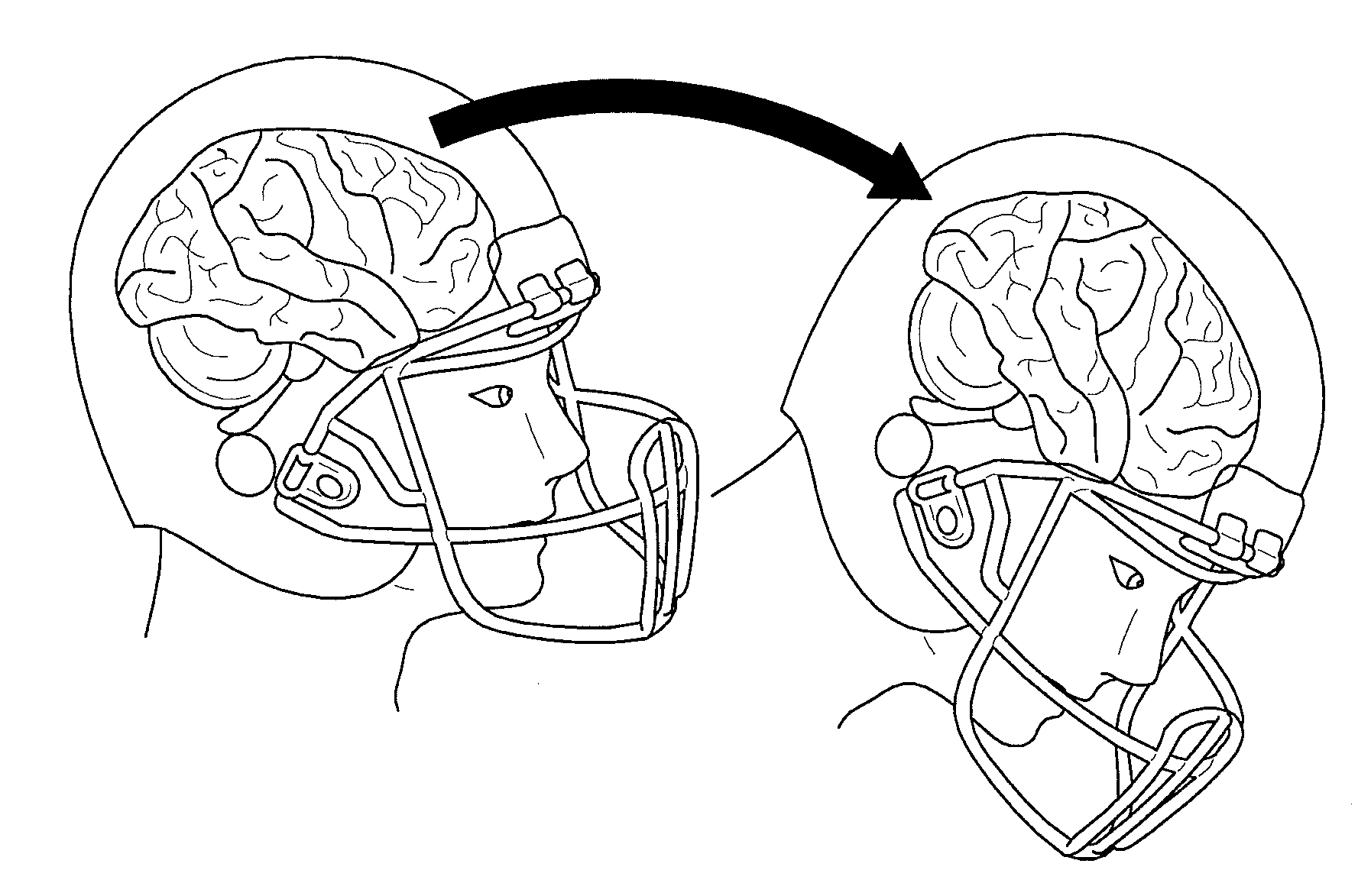
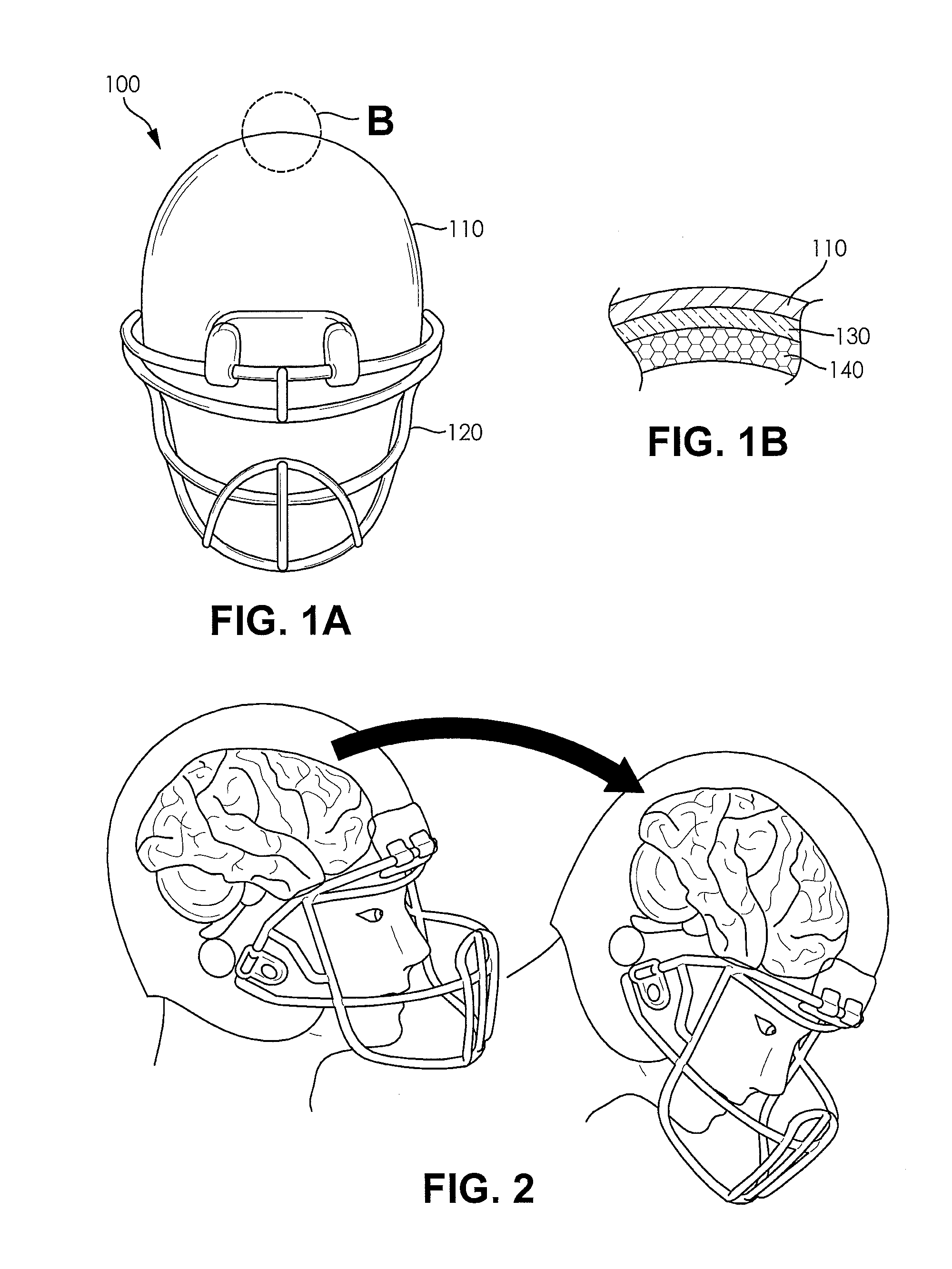
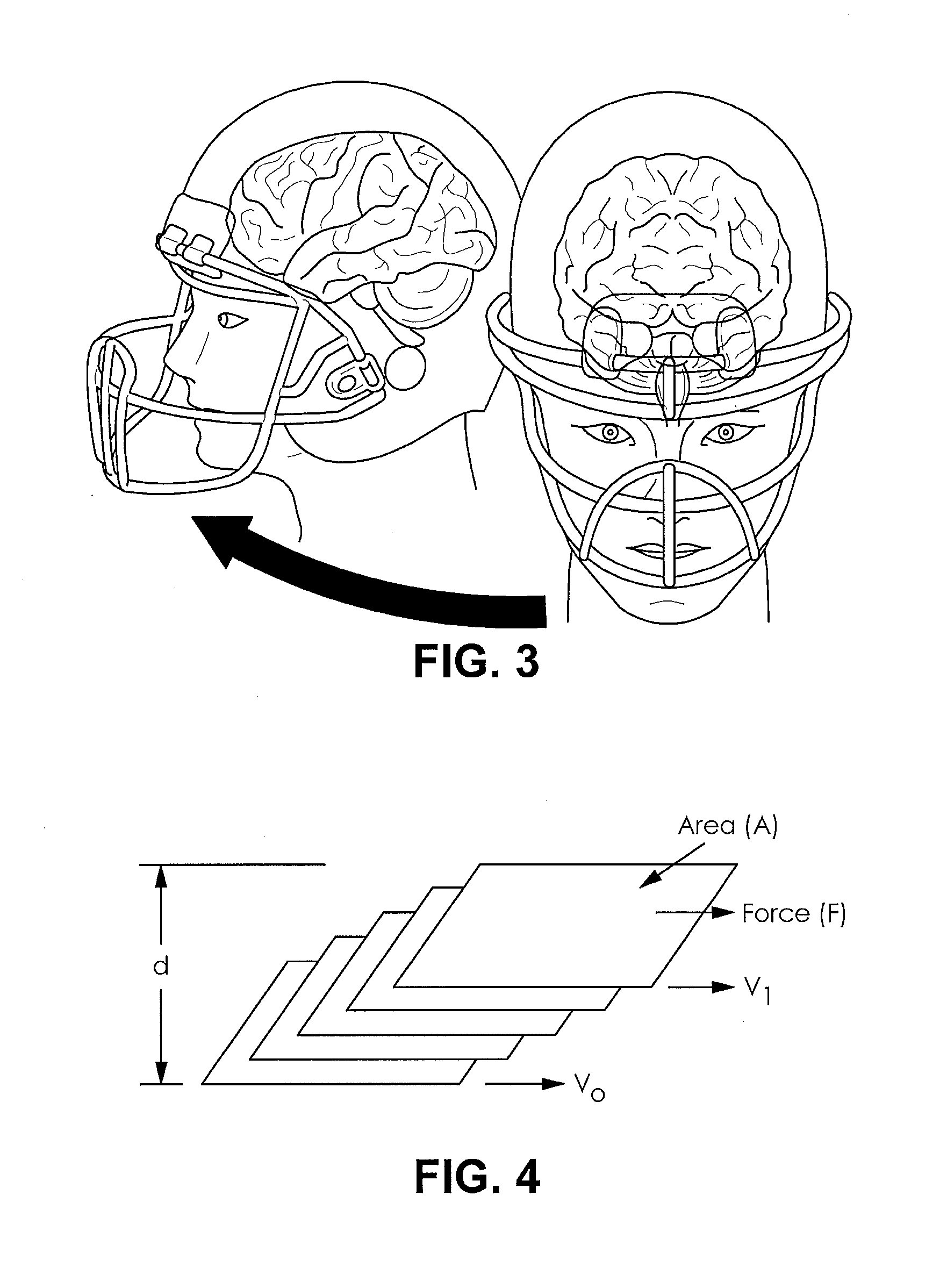
Football helmet liner to reduce concussions and traumatic brain injuries
A composite multi-axial impact protection liner for a helmet is provided that reduces rotational acceleration, rotational strain rate, and rotational strain that cause concussions. In a protective helmet so equipped, one or more layers of fluid polymer, including strain thinning and strain thickening polymers, are positioned between the wearer's head and a hard helmet shell. The liner offers greater injury protection, performance, and personal comfort using the rate dependent and combined effect of strain thinning and strain thickening of fluid polymer layers.
Owner:ZYMPLR LC
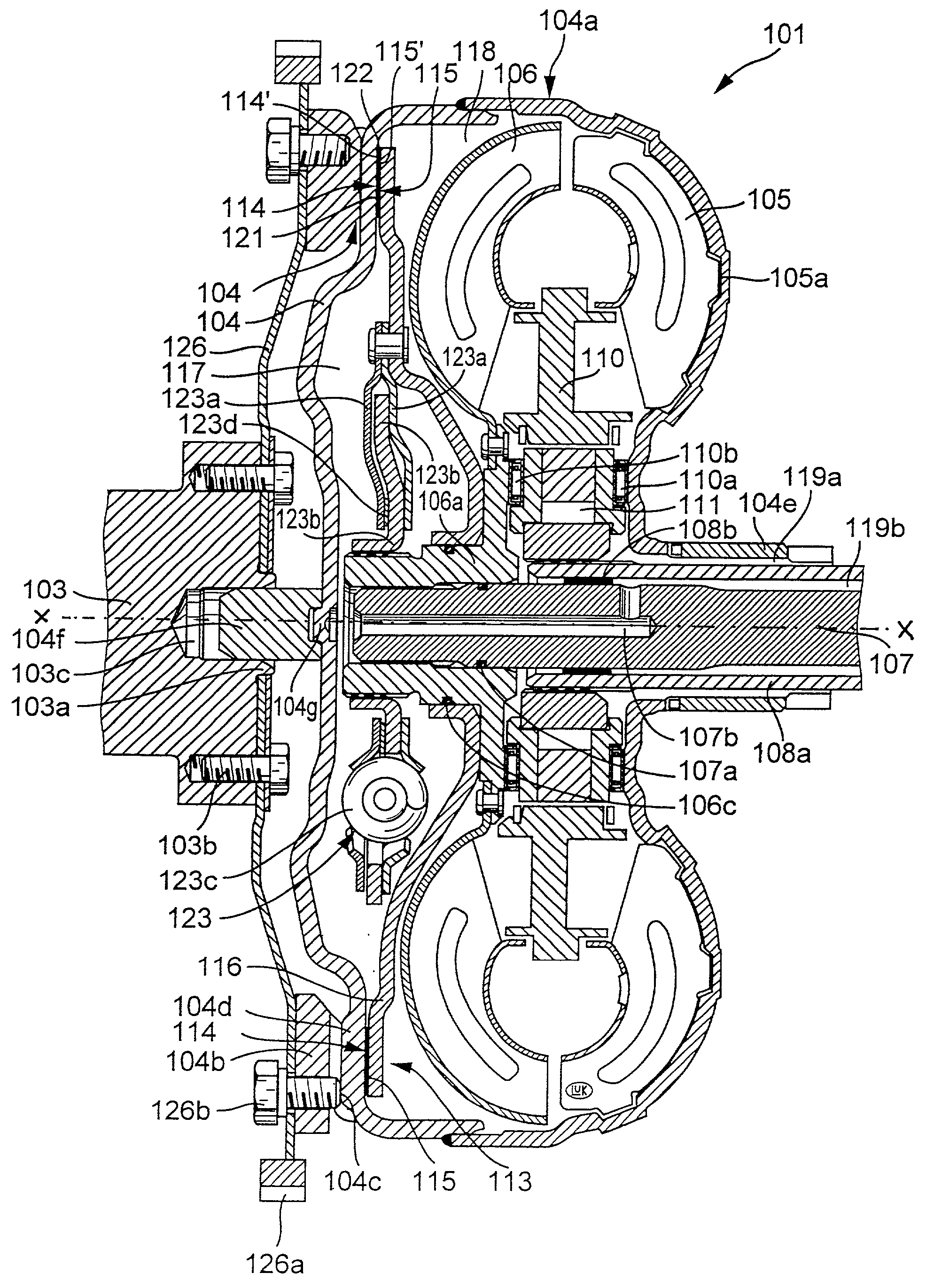
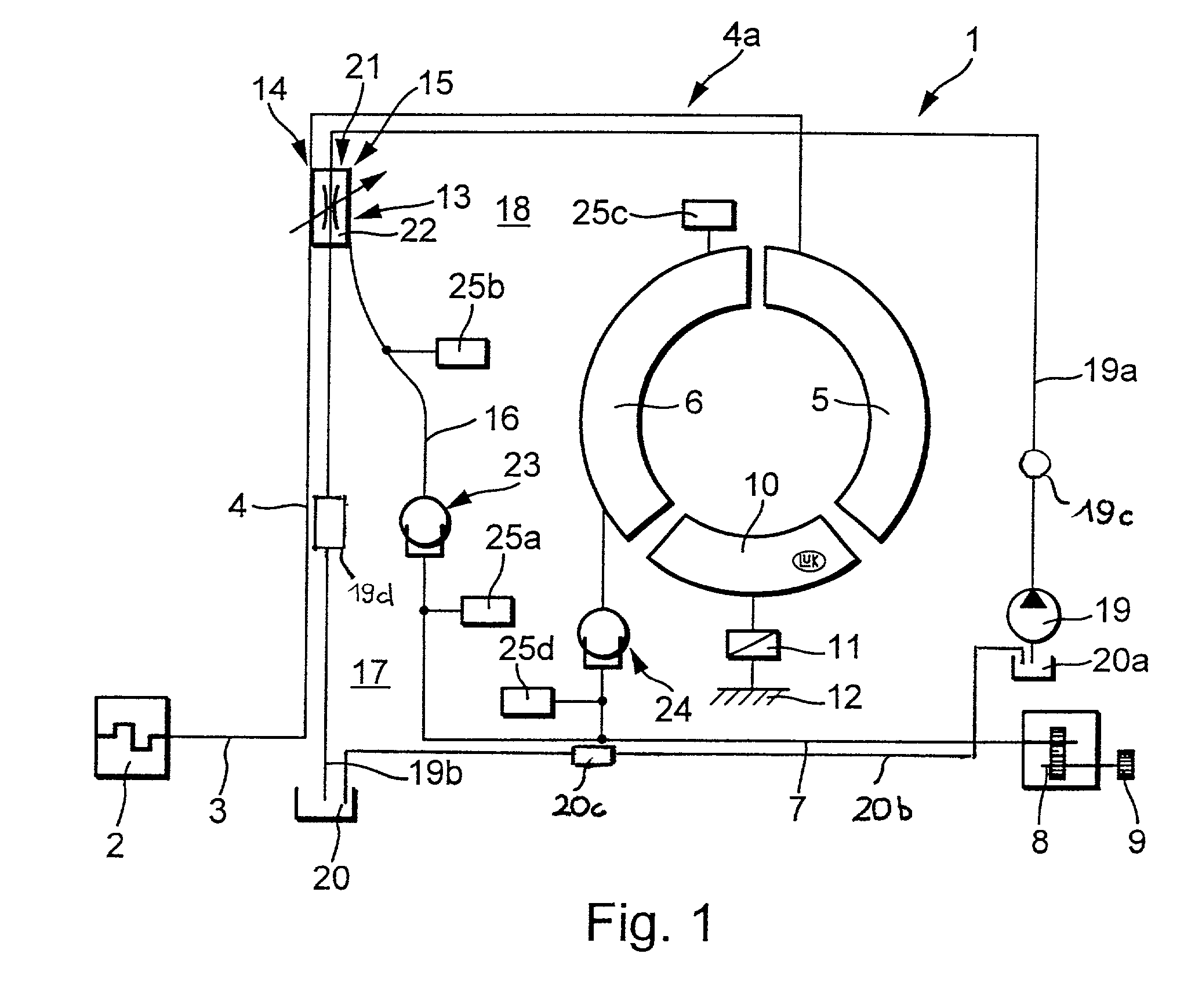
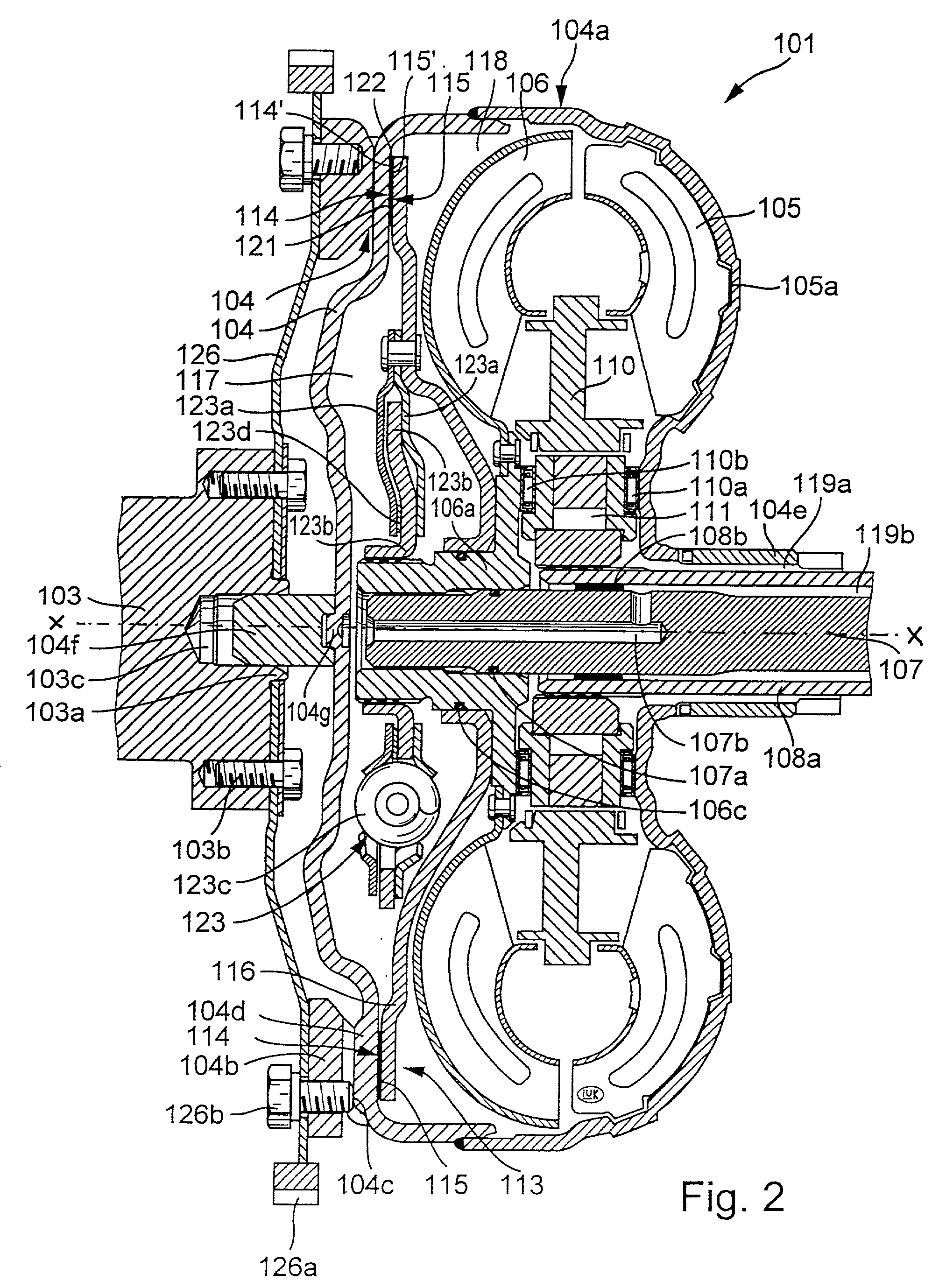
Torque transmitting apparatus
InactiveUS20020027053A1Heat dissipationAvoid damageRotary clutchesFriction clutchesEngineeringHydraulic fluid
A hydrokinetic torque converter with a built-in bypass clutch is provided with an arrangement which regulates the cooling of the clutch at a rate dependent upon the slip between the coaxial driving and driven parts of the clutch, and hence upon the quantity of generated friction heat. The cooling unit for the driving and / or driven part of the clutch can employ, for example, one or more pumps; a supply of a substance which changes its aggregate state from liquid to gaseous or from solid to flowable in response to heating, and vice versa in response to cooling; one or more porous washers in the path for the flow of hydraulic fluid between the customary plenum chambers provided in the housing of the torque converter to move a piston of the driven part of the clutch into and from frictional engagement with the housing; and / or a system of recesses, grooves, channels and / or other passages serving to convey fluid between the chambers at a rate which is higher or highest when the clutch operates with maximum slip. Such rate can decrease to zero when the torque converter is idle or the clutch is fully engaged to operate without slip.
Owner:LUK LAMELLEN & KUPPLUNGSBAU BETEILIGUNGS KG
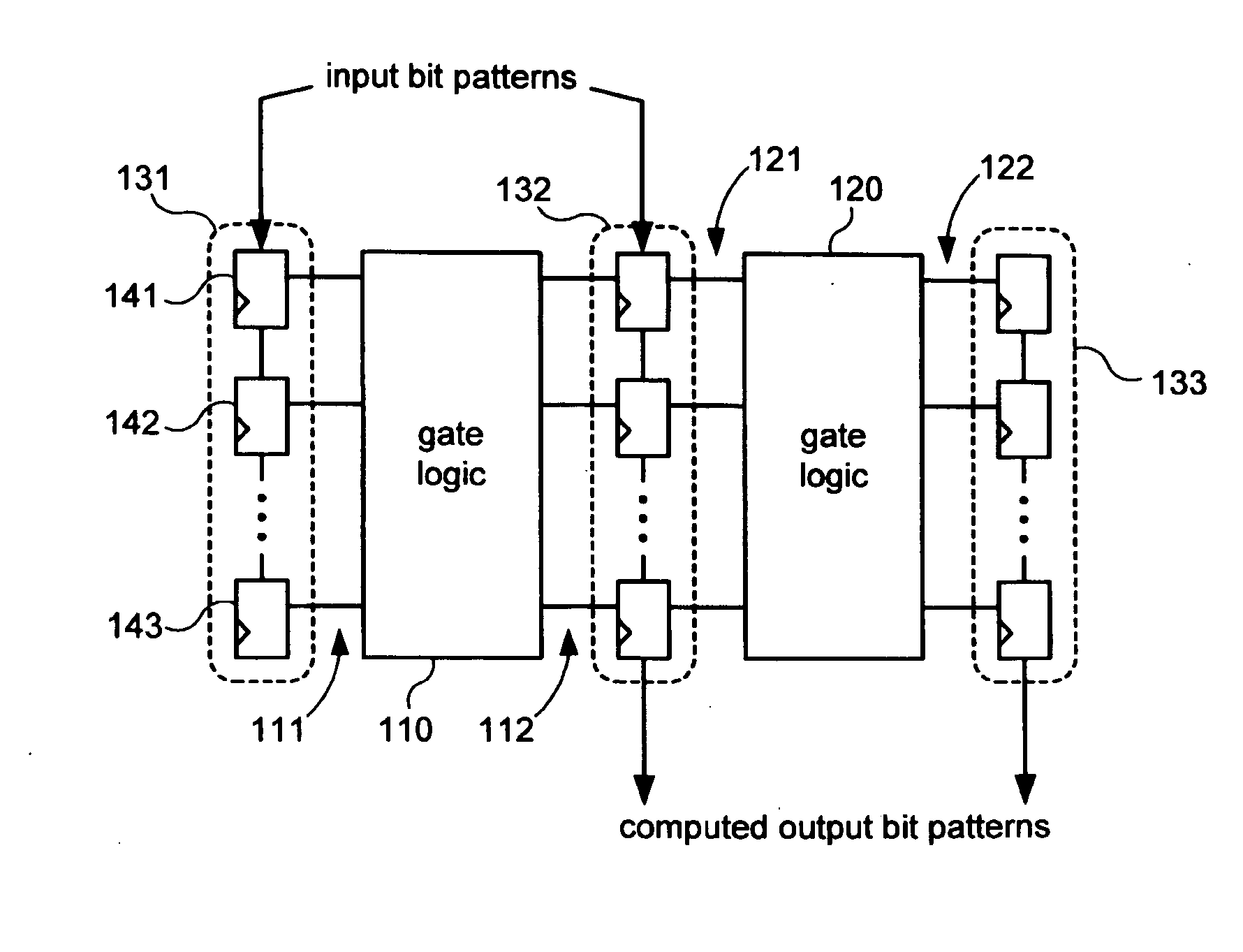
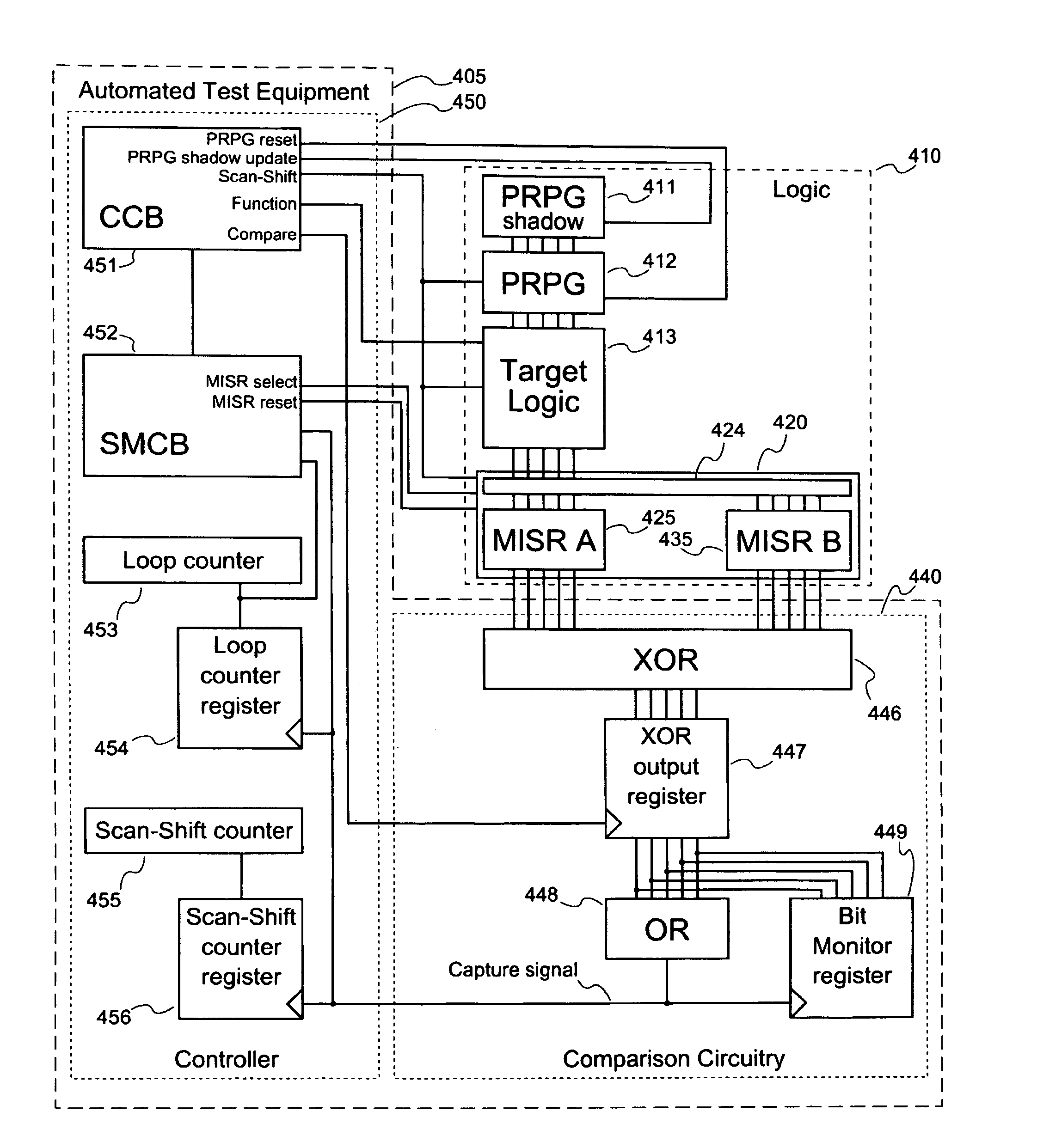
Systems and methods for diagnosing rate dependent errors using LBIST
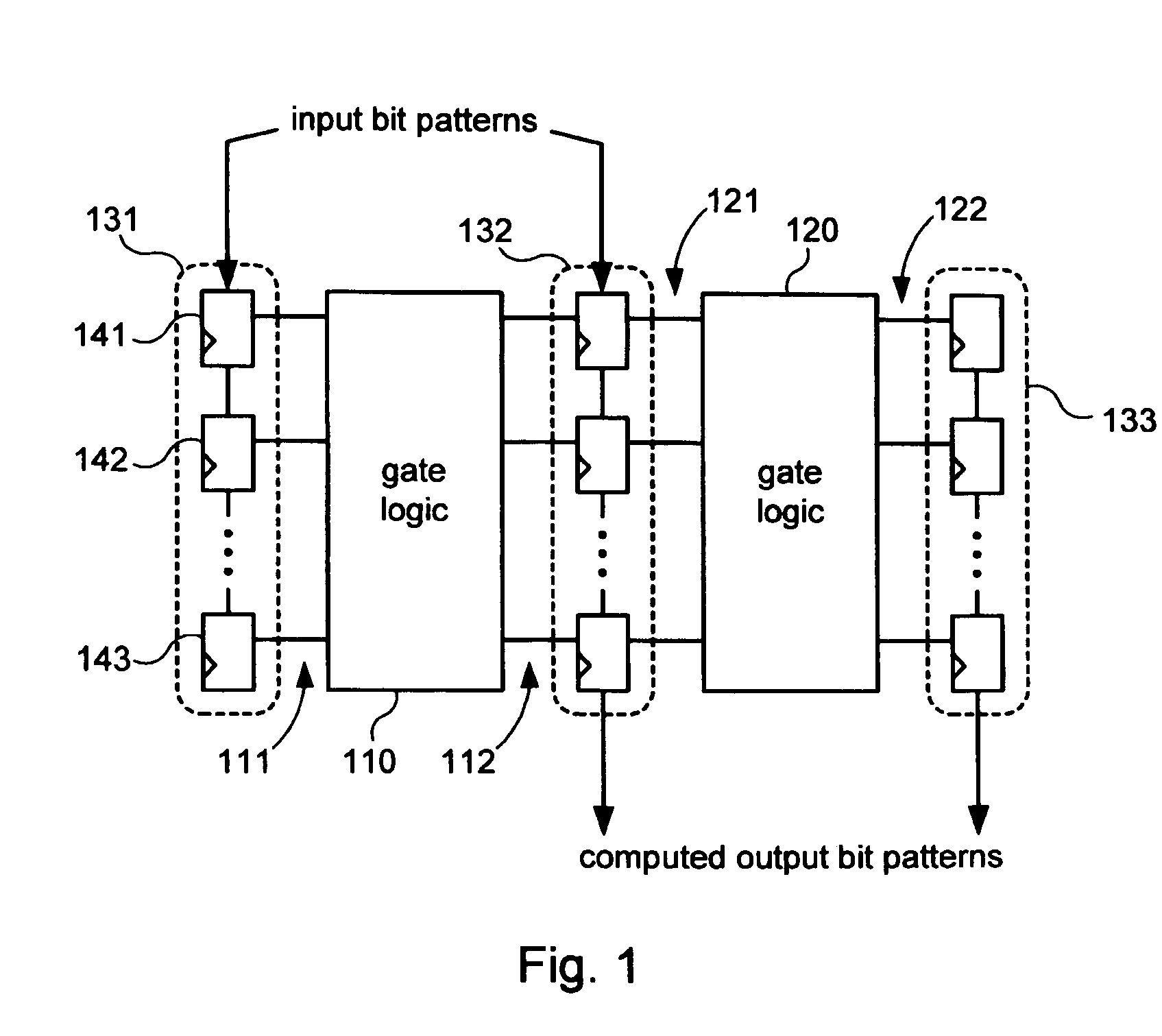
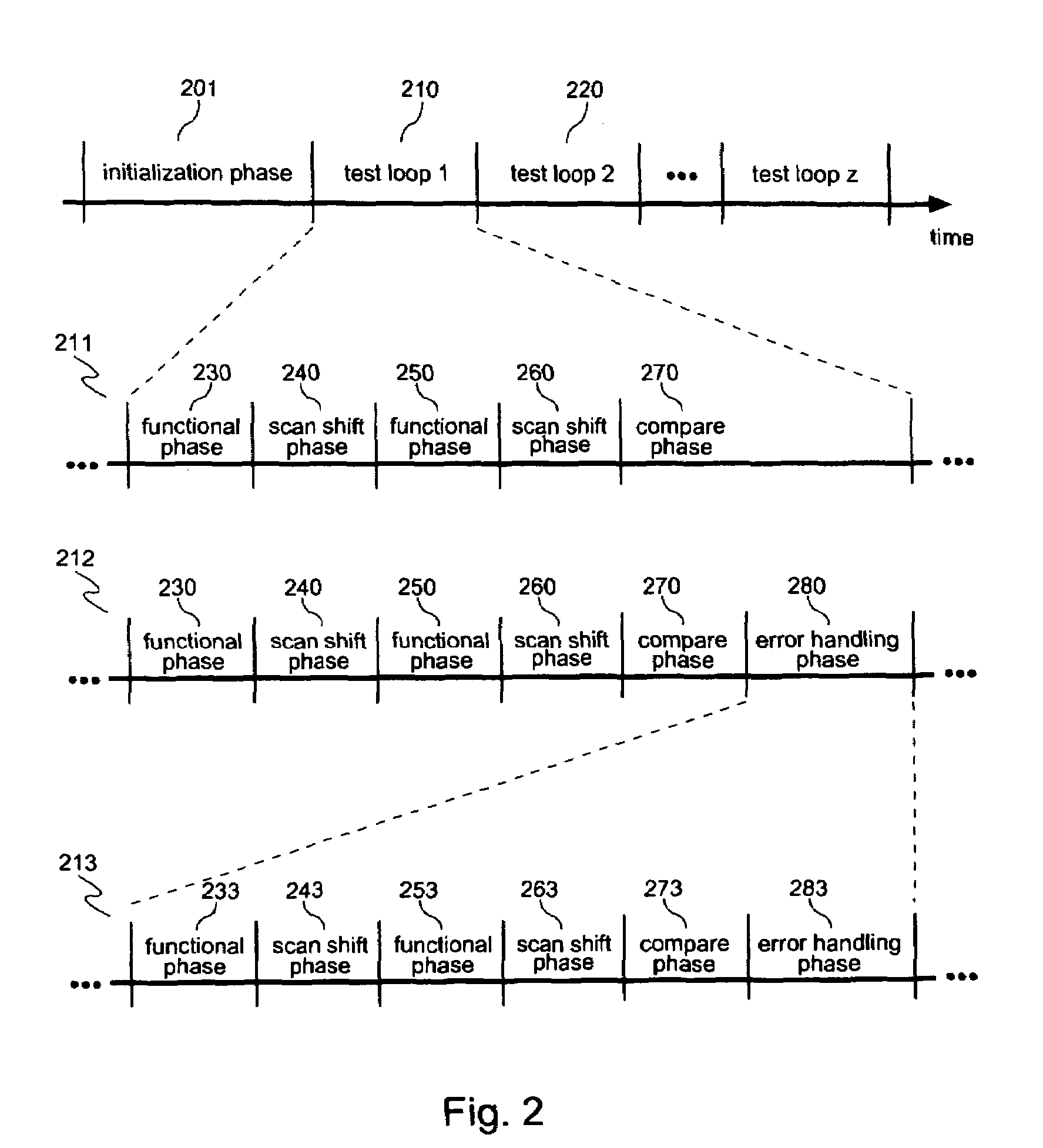
InactiveUS20070050693A1Electronic circuit testingError detection/correctionLogic built-in self-testComputer science
Systems and methods for performing logic built-in self-tests (LBISTs) to detect “at-speed” errors in a digital circuit. In one embodiment, an input bit pattern is propagated through target logic of the digital circuit and captured in scan chains at a normal operating speed to produce a first output bit pattern. This is repeated with the first input bit pattern at a lower test speed to produce a second output bit pattern. Differences between the first and second output bit patterns are then detected to determine whether operation of the digital circuit at the normal operating speed causes errors that are not generated at the lower test speed.
Owner:KK TOSHIBA
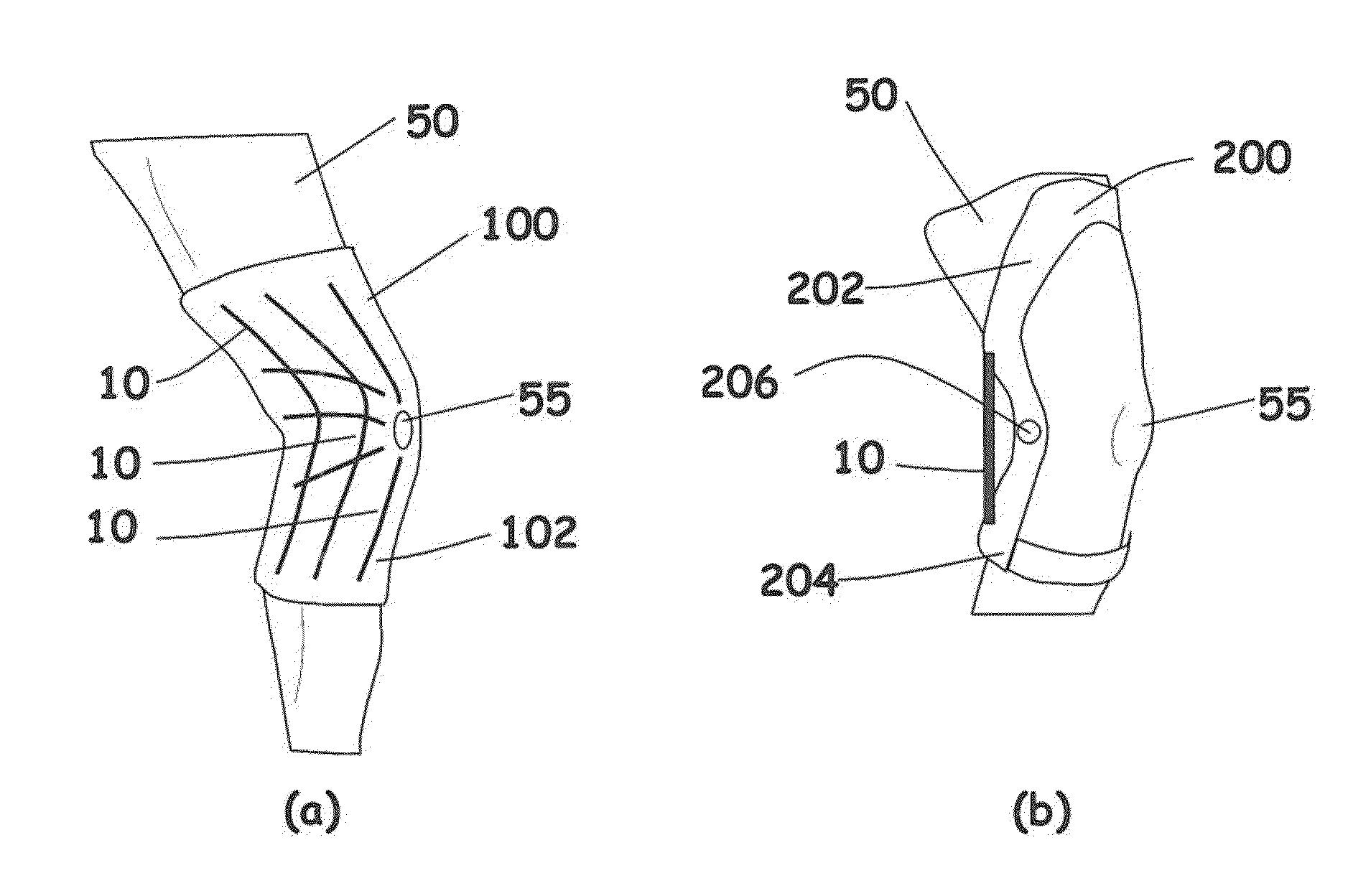
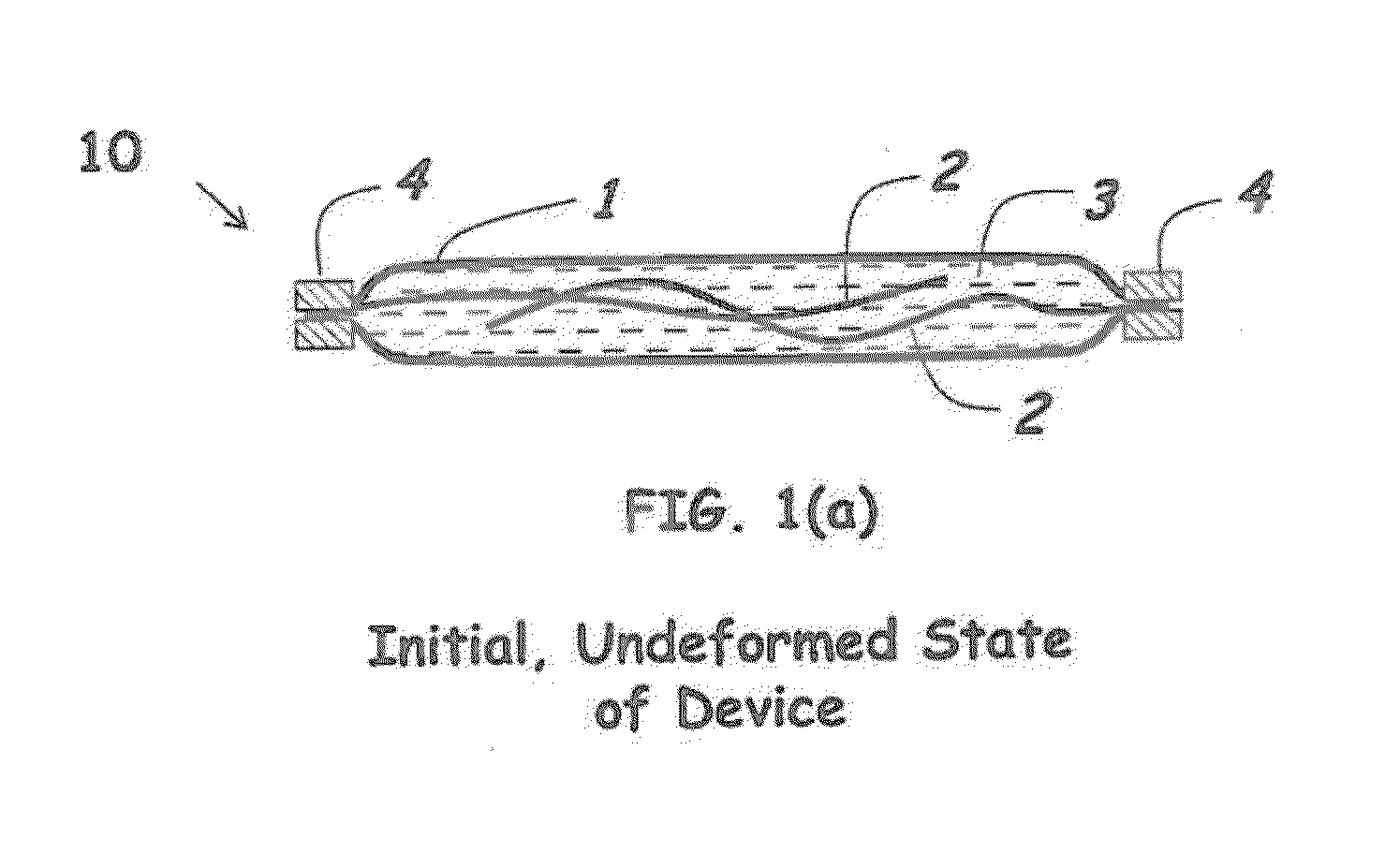
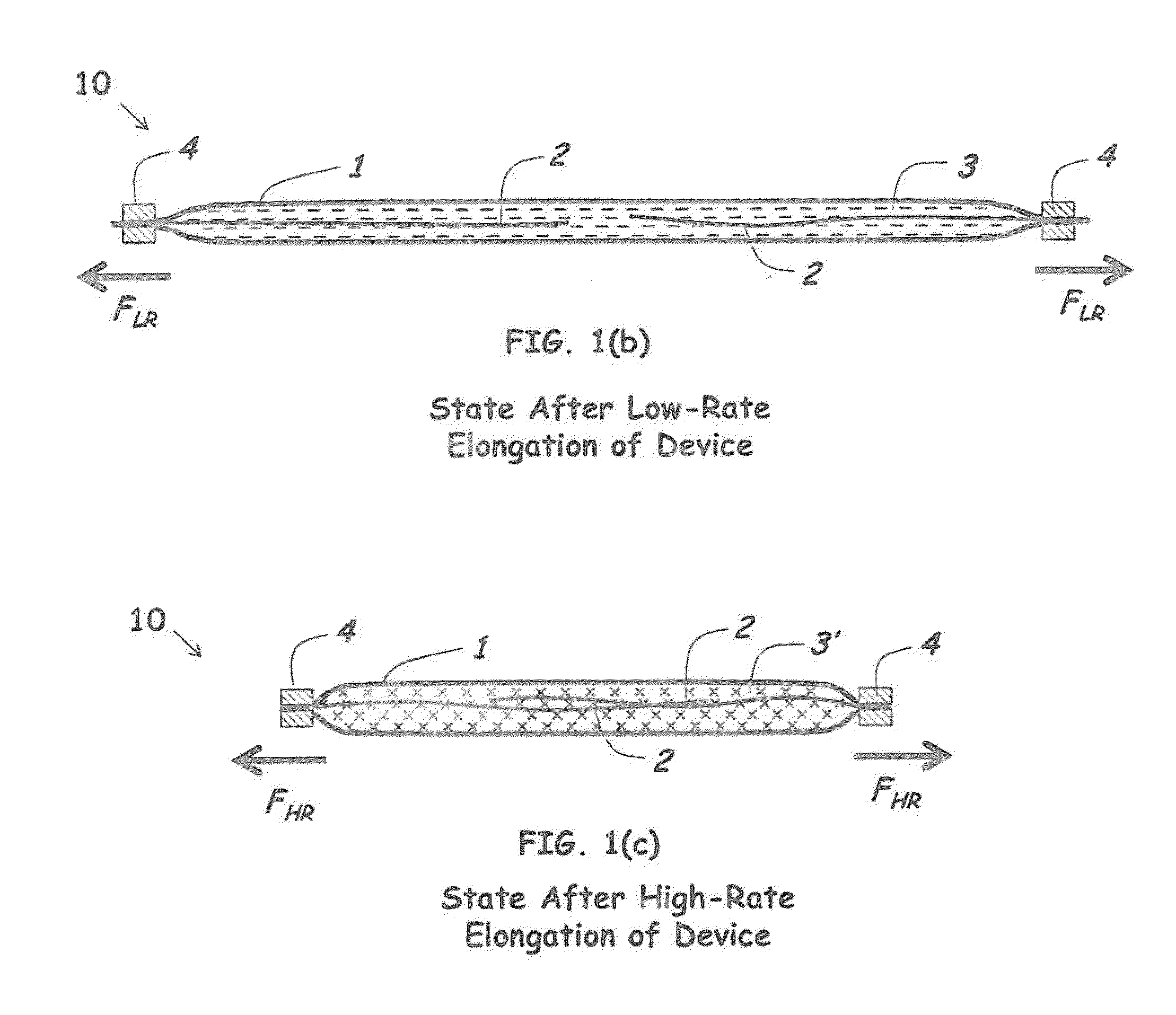
Rate-responsive, stretchable devices
ActiveUS20140015176A1High elongationHigh energySpringsMedical scienceBiomedical engineeringRate dependent
Rate-dependent, elastically-deformable devices according to various embodiments can be stretched and recovered at low elongation rates. Yet they become stiff and resistive to stretching at high elongation rates. In one embodiment, a rate-dependent, elastically-deformable device includes an elastically-deformable confinement member; one or more filaments placed inside the elastically-deformable confinement member; and a fluid that substantially fills the remaining volume inside the elastically-deformable confinement member. The resistance force to extension of the device is designed to increase as the extension rate of the device increases. At low elongation rates the filaments can readily slide past each other. At high elongation rates, the fluid transforms to a less flowable material that greatly increases the force and energy required for elongation; or transforms to a non-flowable material that resists elongation. The devices thus can be stretched and recovered at low elongation rates, but become extremely stiff and resistive to stretching at high elongation rates.
Owner:ARMY US SEC THE THE
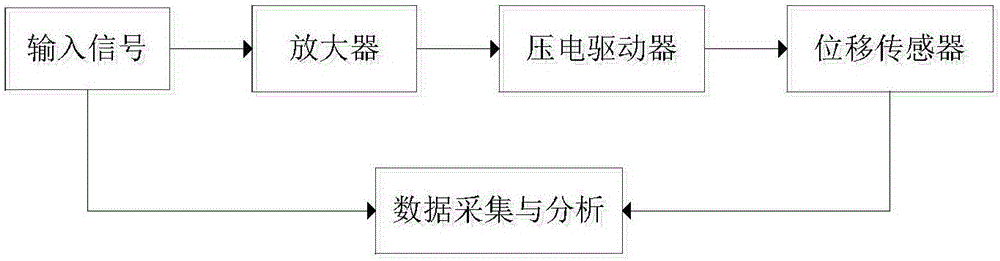
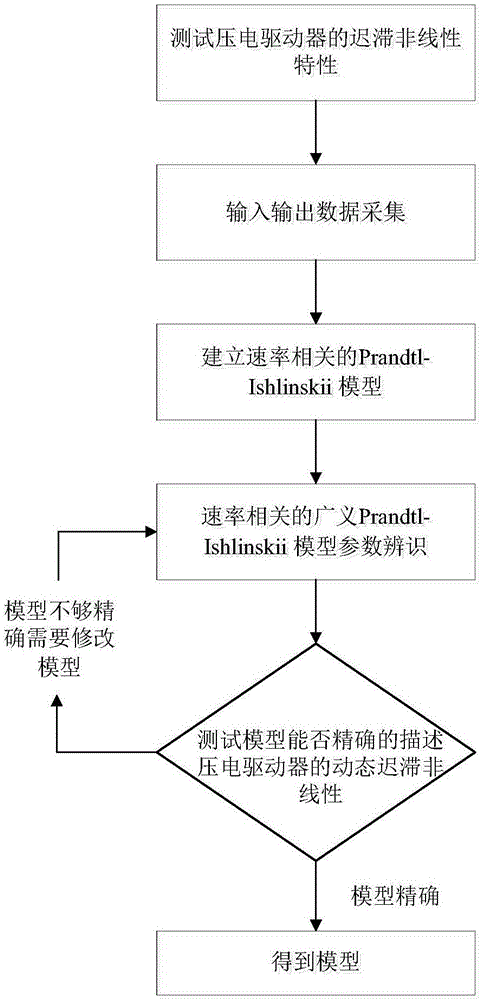
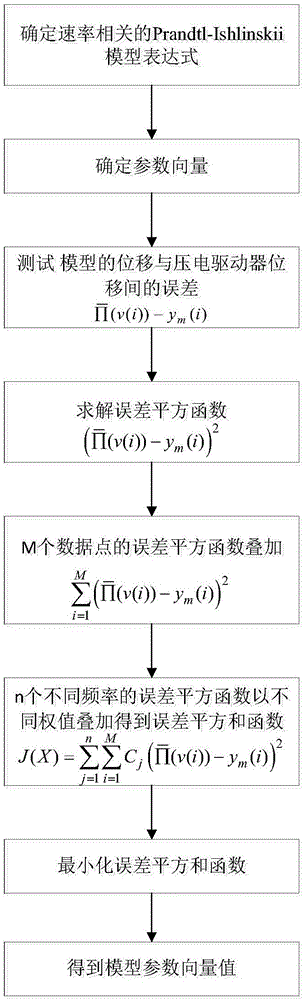
Nonlinear inverse control method used for dynamic hysteresis compensation of piezoelectric actuator
ActiveCN106707760AAccurately describe dynamic hysteresis characteristicsHigh positioning accuracyAdaptive controlHysteresisLoop control
The invention discloses a nonlinear inverse control method used for dynamic hysteresis compensation of a piezoelectric actuator. Nonlinear inverse control of the piezoelectric actuator is performed based on a Prandtl-Ishlinskii model by aiming at the problem that most models cannot perform accurate inverse analysis for the modeling difficulty of a dynamic hysteresis system; a dynamic critical value related to the input frequency is established to obtain a rate-dependent play operator, and the rate-dependent play operator is combined with a density function so as to obtain a rate-dependent Prandtl-Ishlinskii model; a hysteresis main ring is measured under different input frequencies so as to determine model parameters; the inverse parameters of the model are inversely solved by solving an initial load curve so as to obtain a rate-dependent Prandtl-Ishlinskii inverse model; and the Prandtl-Ishlinskii model and the inverse model thereof are used for an open-loop control system so as to compensate the hysteresis nonlinear property of the piezoelectric actuator. The experiment proves that the rate-dependent Prandtl-Ishlinskii model can accurately describe the hysteresis nonlinearity of the piezoelectric actuator and the rate-dependent Prandtl-Ishlinskii inverse model enhances the positioning and control precision of a hysteresis nonlinear system.
Owner:NANJING UNIV OF SCI & TECH
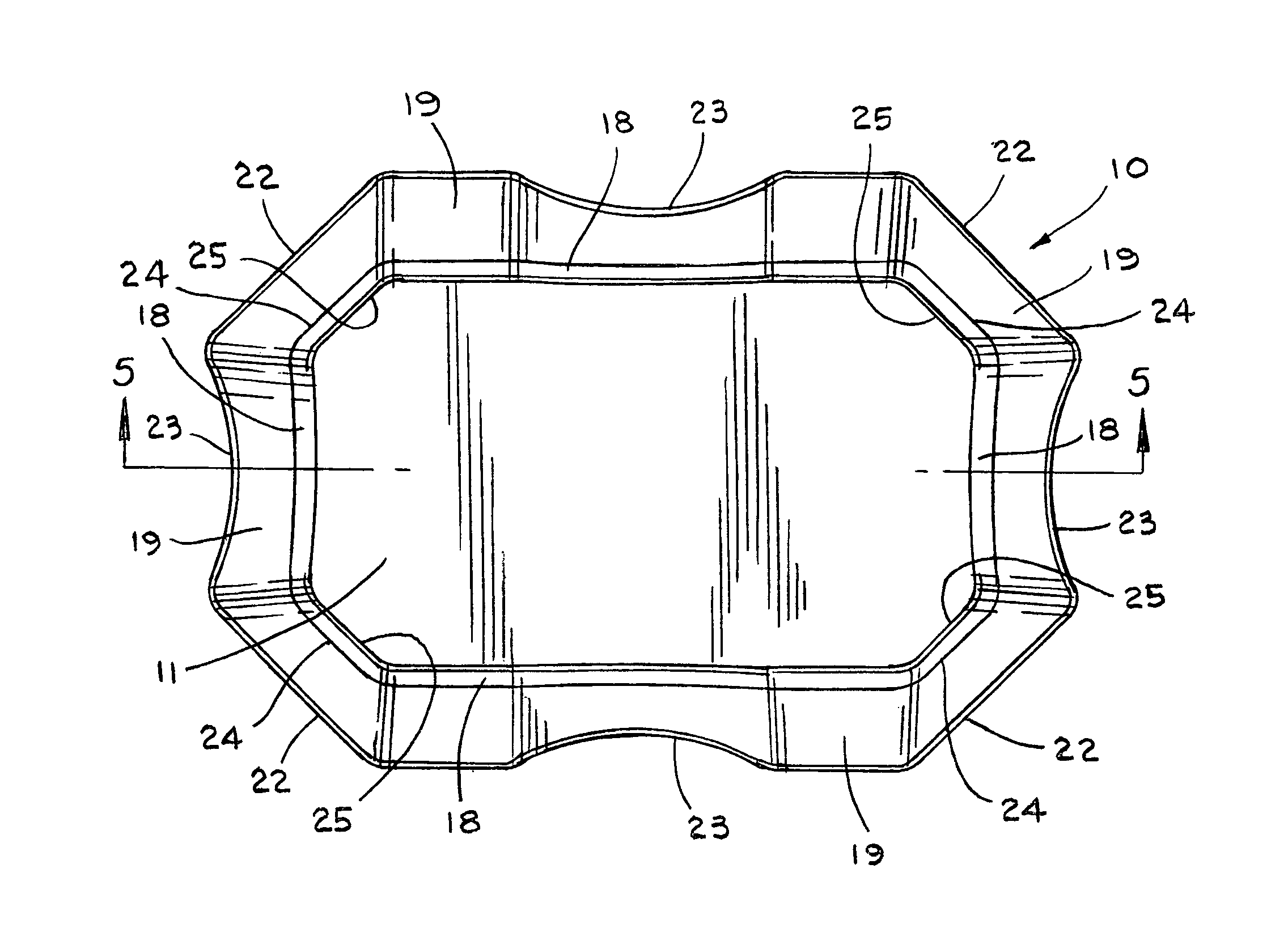
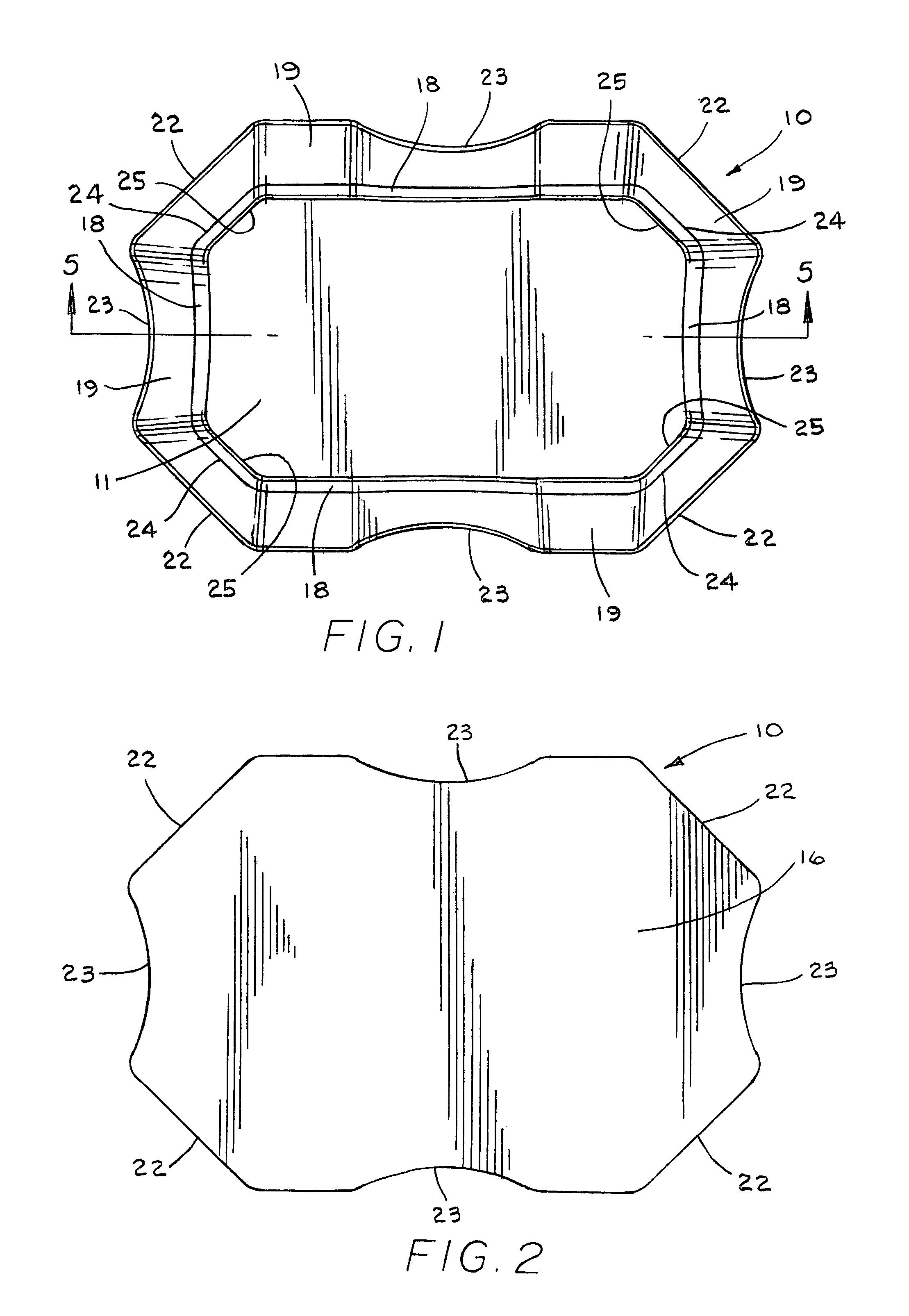
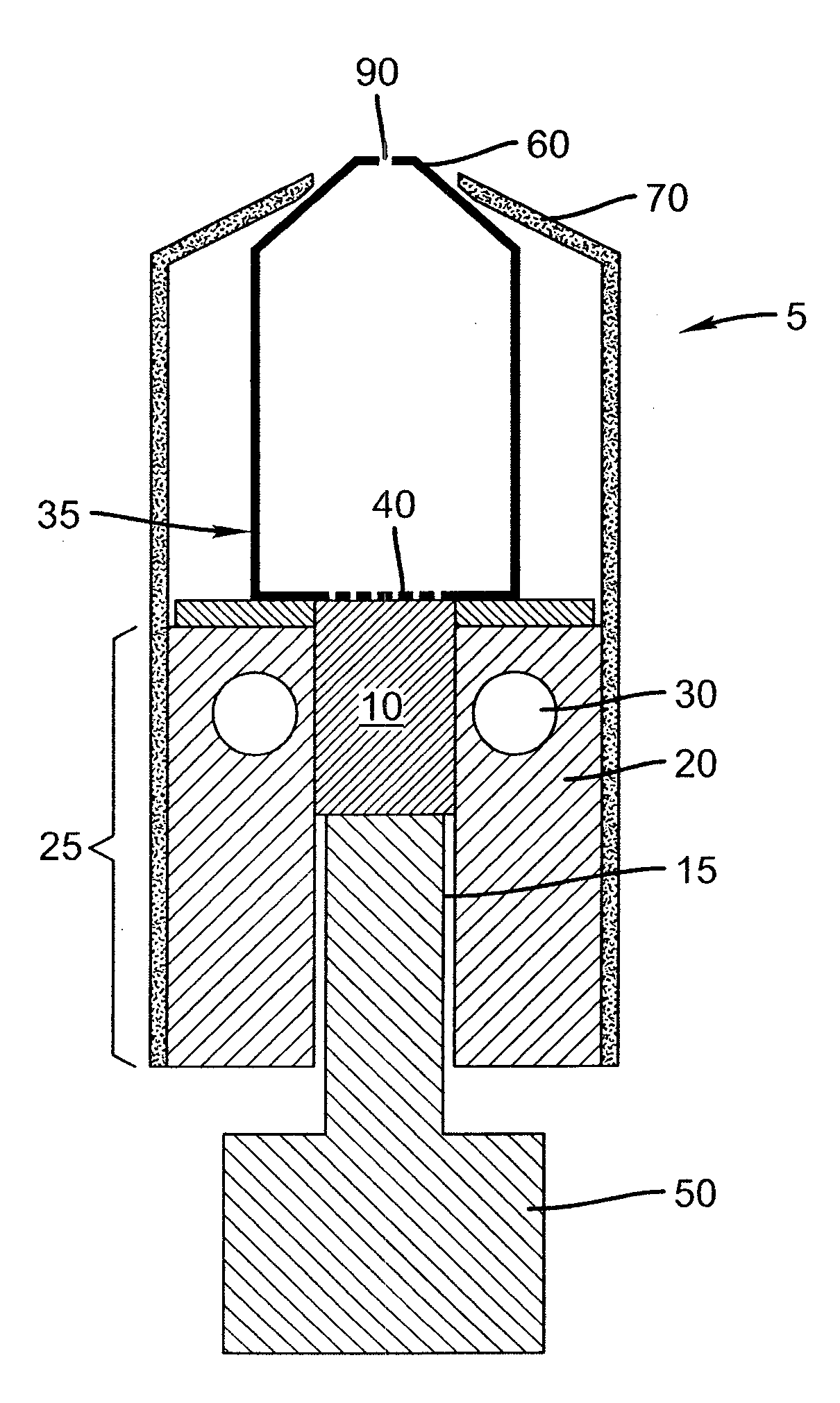
Pad for helmet or the like
InactiveUS8001622B1Simple and inexpensive to manufactureProvide comfortHatsHeadwear capsEngineeringMechanical engineering
A pad (10), a plurality of which can be positioned in a helmet (21) or the like for comfort and protection, includes a first generally rectangular fabric material (11) having truncated corners (25). A first generally rectangular foam material (12) having truncated corners (24) includes a top surface, a bottom surface, and side surfaces (18), the top surface being attached to the first fabric material (11). A second generally rectangular foam material (14) having truncated corners (22) includes a top surface, a bottom surface, and side surfaces (19) having a scallop (23), the top surface being attached to the bottom surface of the first foam material (14). A second generally rectangular fabric material (16) having truncated corners (22) and sides having a scallop (23) is attached to the bottom surface of the second foam material (14). The side surfaces (18, 19) of the first and second foam materials (12, 14) are exposed. The first foam material (12) is an open-celled polyurethane, and the second foam material (14) is an impact rate dependent polyurethane having a thickness at least as great or greater than the thickness of the first foam material (12).
Owner:REMINGTON PROD INC
Image compression processing device, image compression processing method, and image compression processing program
InactiveUS20070110328A1Easy to useQuick noteTelevision system detailsPicture reproducers using cathode ray tubesImage compressionImage signal
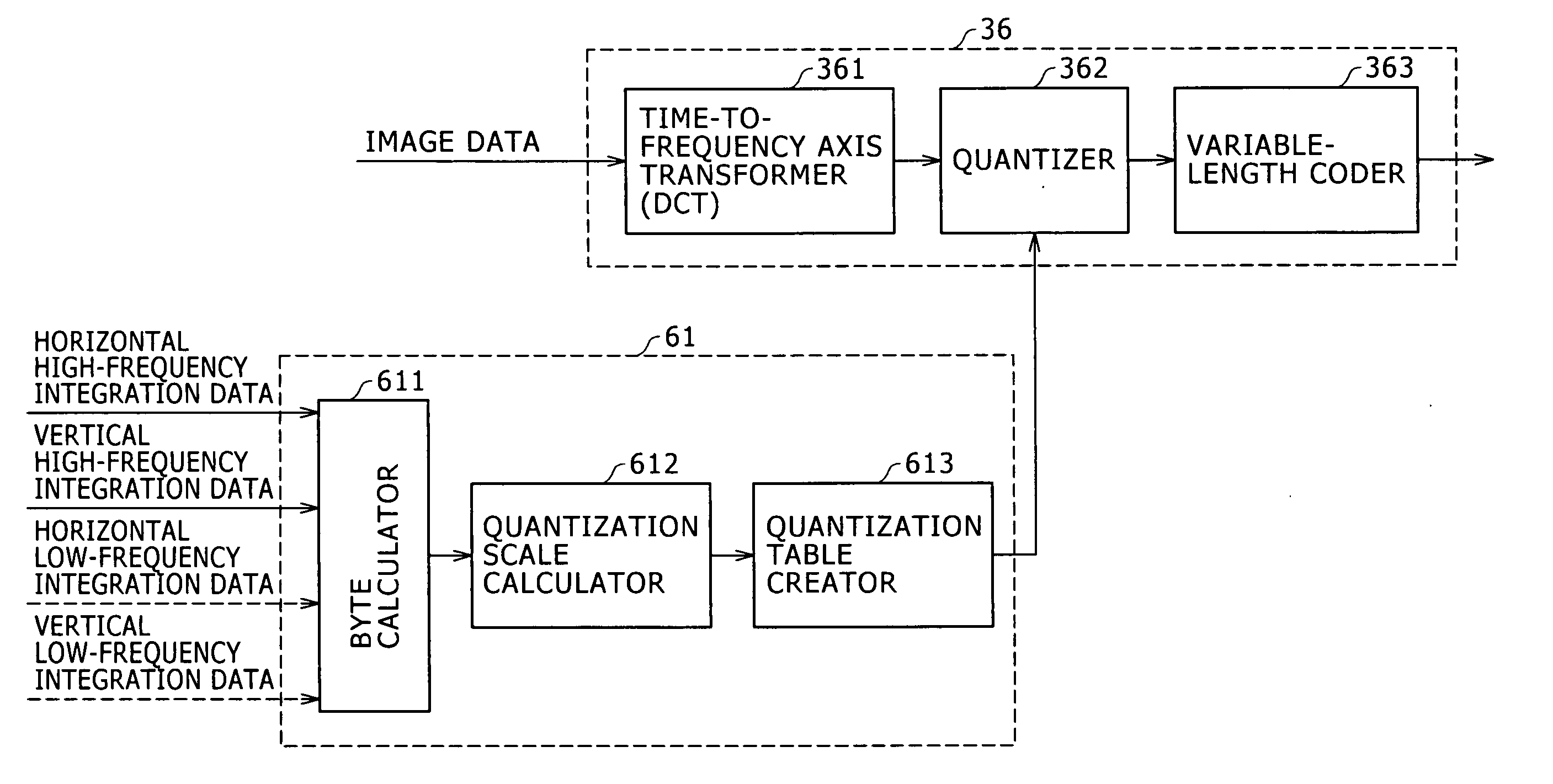
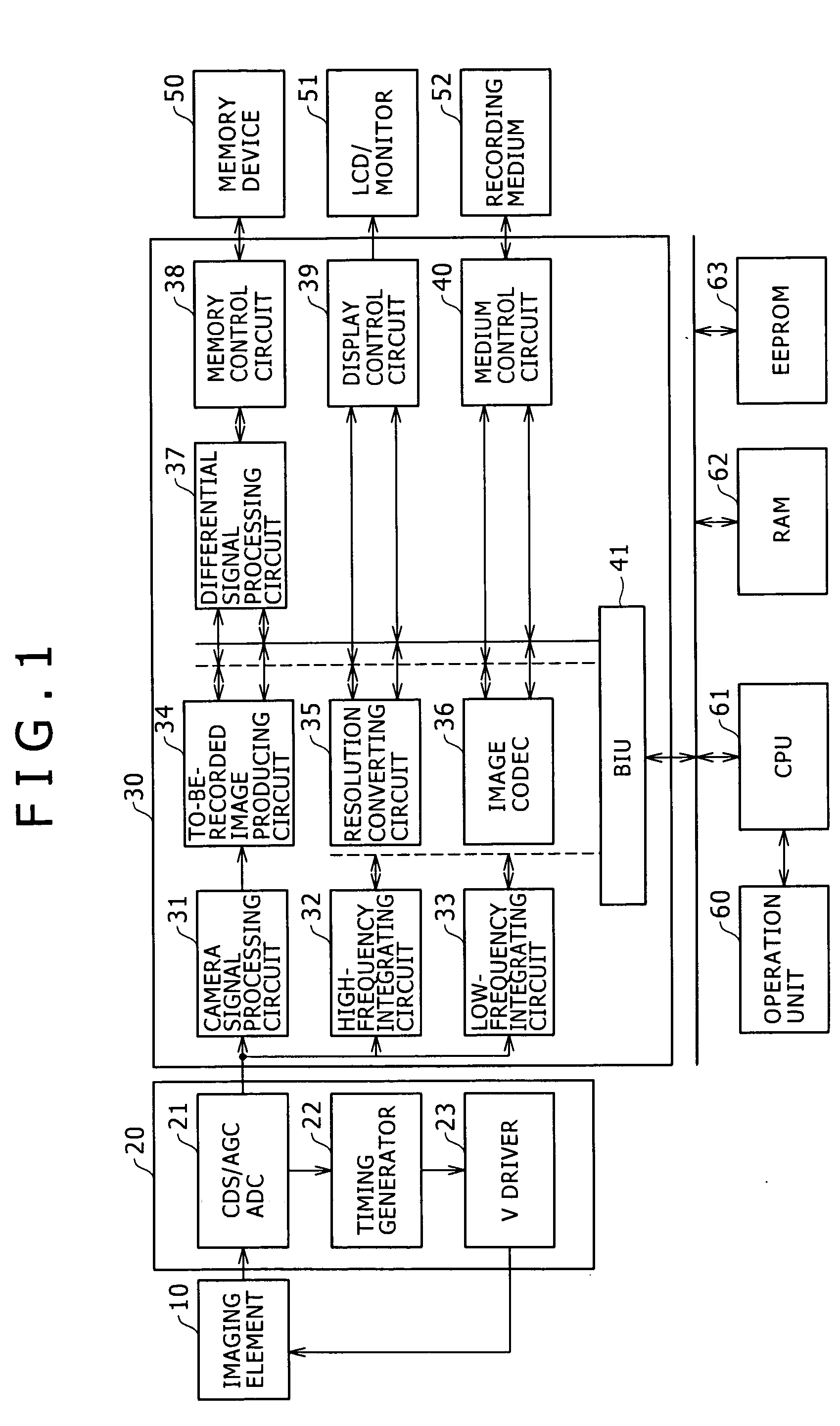
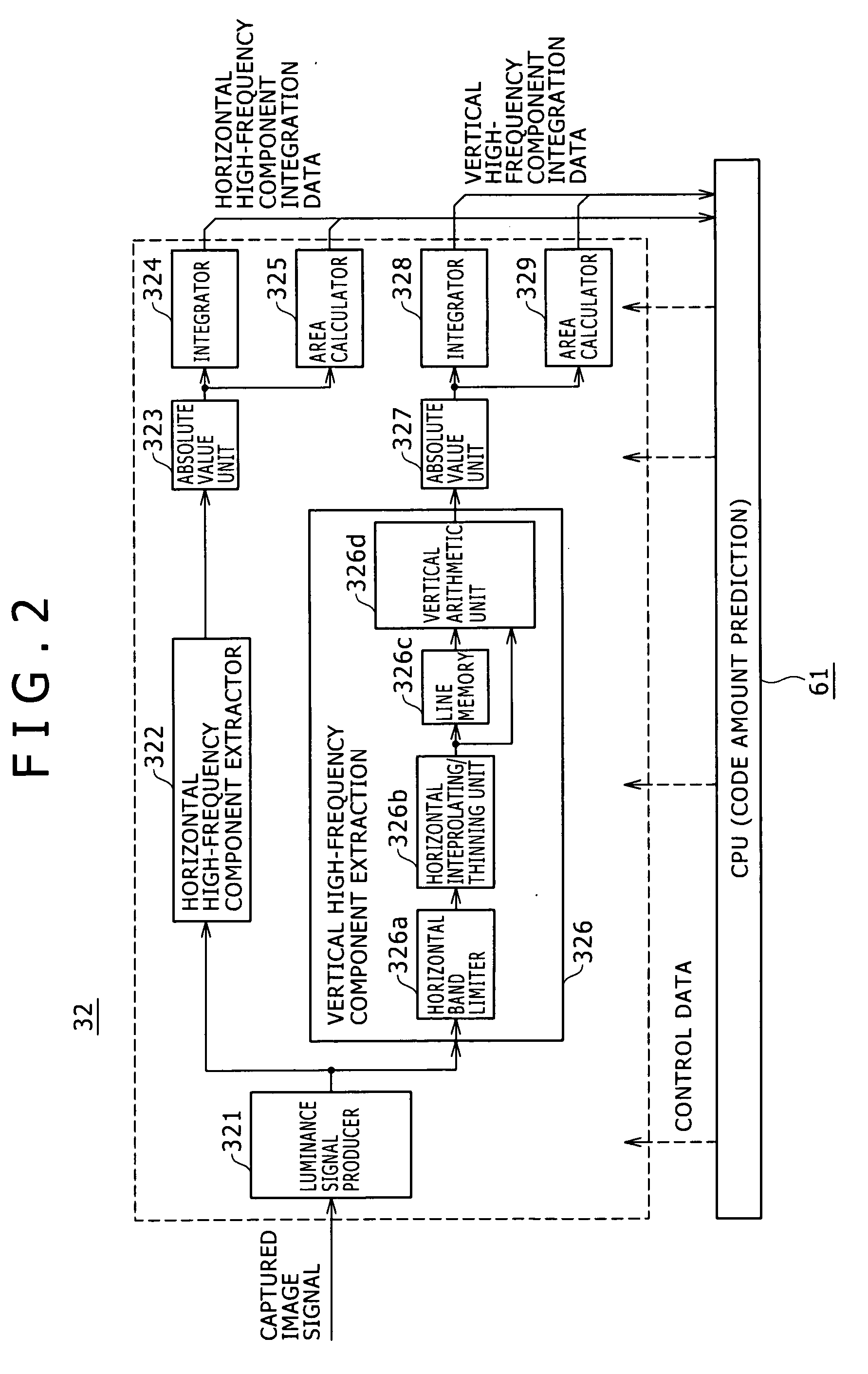
A high-frequency integrating circuit 32 detects characteristics of horizontal high-frequency components and vertical high-frequency components of the image formed by a processing-target image signal. Based on the detection result, a CPU 61 obtains the number of bits of the after-compression-coding data of the image signal, and calculates the compression rate dependent upon the number of bits. The CPU 61 controls an image codec 36 so that the processing-target image signal is compression-coded through only one time of compression coding processing by use of the calculated compression rate. This configuration allows the image compression processing (compression coding) to be rapidly executed with high accuracy.
Owner:SONY CORP
Methods and Devices for Estimating a Level of Use of a Communication Network and for Adapting a Level of Subscription to Multicast Sessions
InactiveUS20110013538A1Easily and efficiently evaluateWithout perturbingError preventionTransmission systemsData streamNetwork link
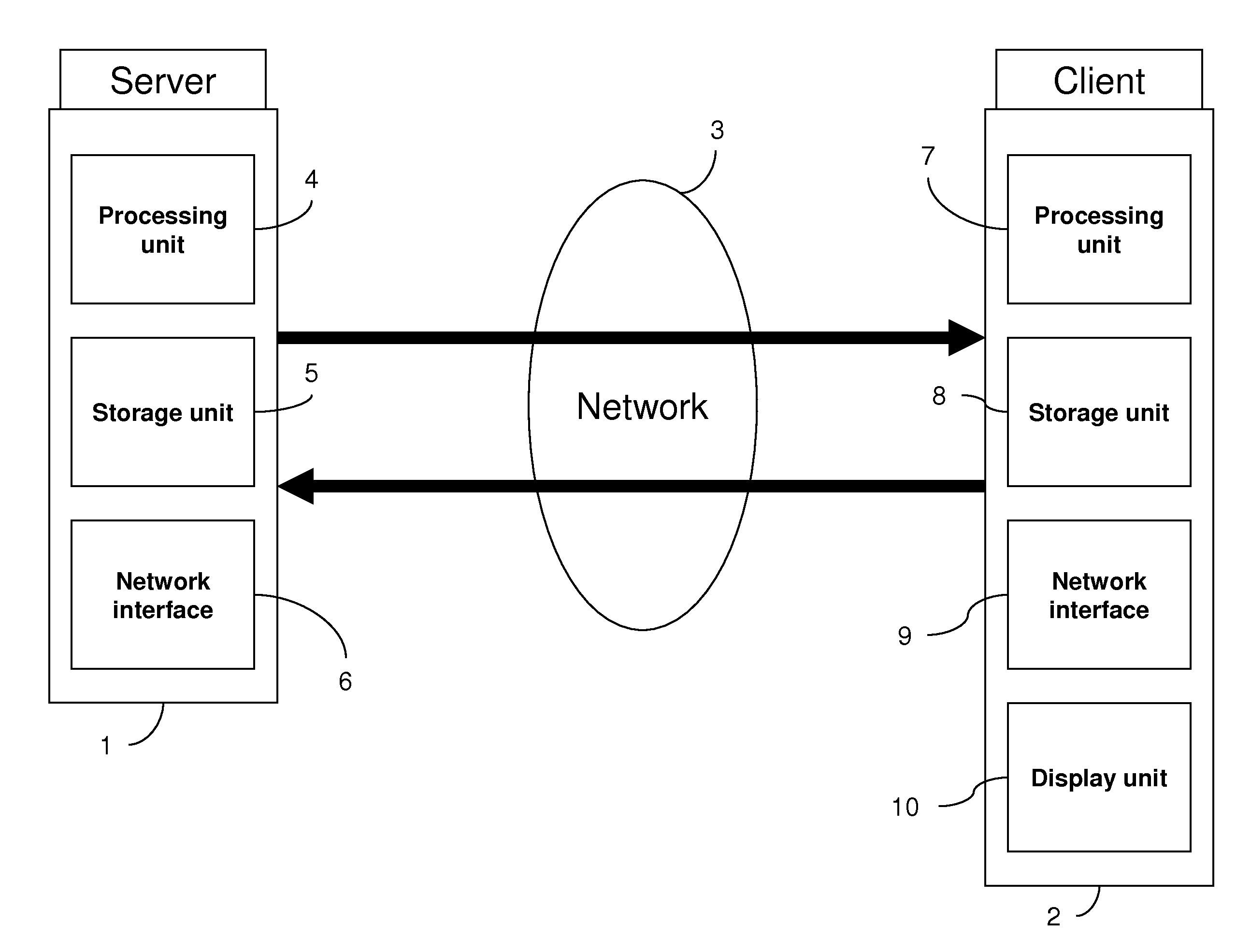
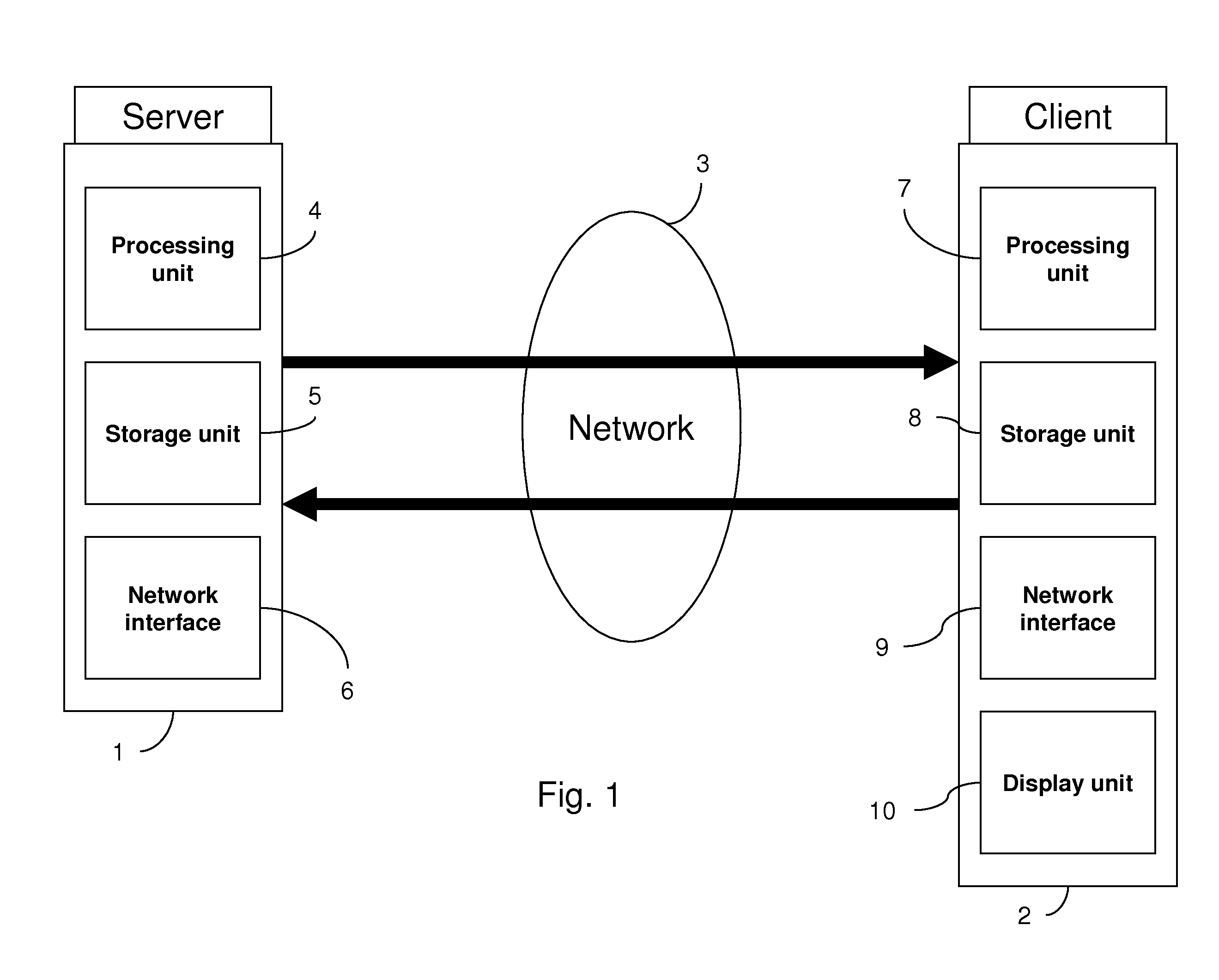
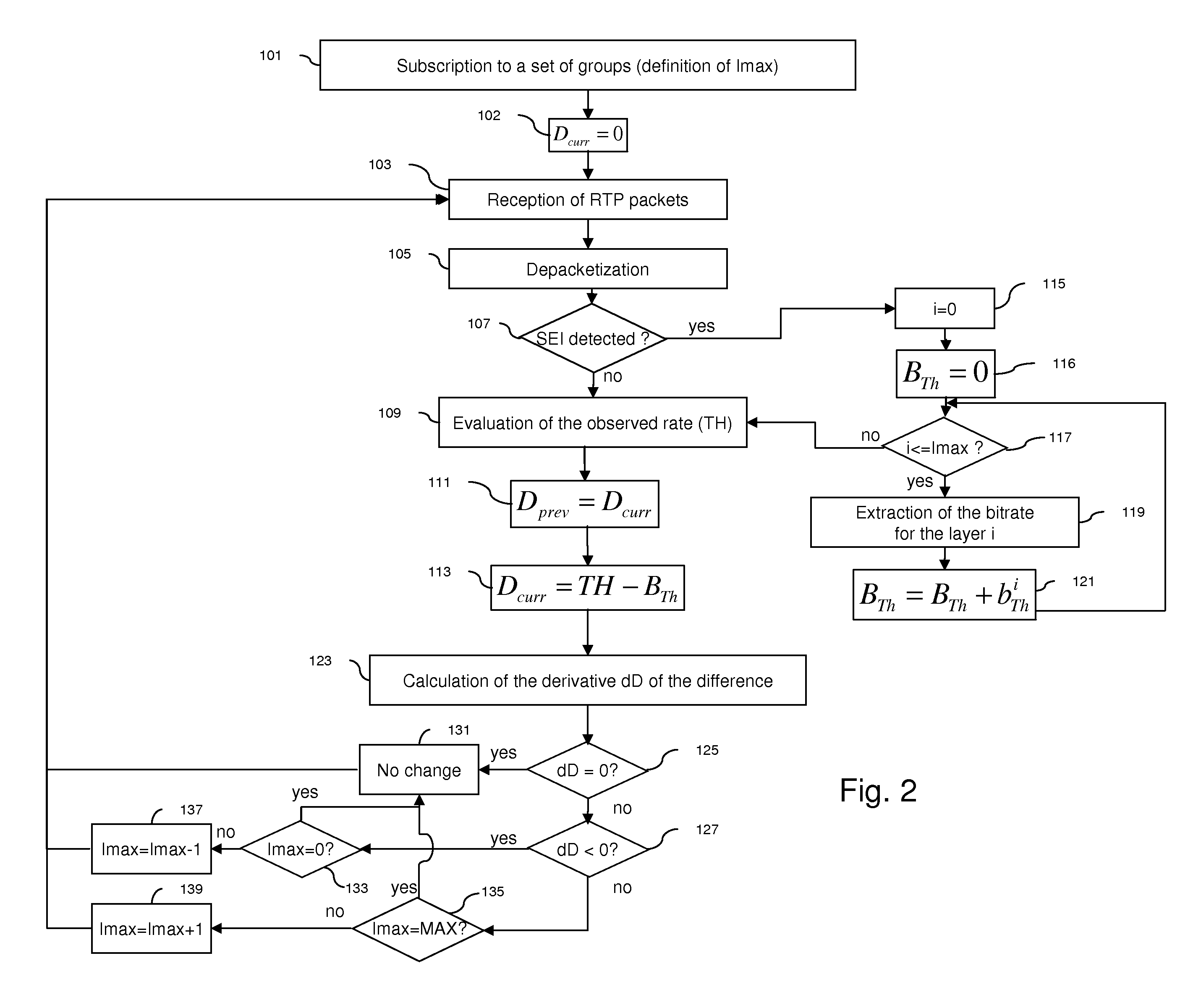
The invention in particular concerns a method and a device for estimating a level of use of a communication network linking a server and at least one client, said communication network being used to transmit a data stream at a transmission rate corresponding to the temporal frequency of said data stream, said data stream comprising at least one item of information characterizing a theoretical rate dependent on said transmission rate of said data stream. After having obtained (121) said theoretical rate of said data stream, the real reception rate of at least one part of said data stream is evaluated (109). Said theoretical rate and said evaluated rate are then compared (113) to estimate said level of use of said communication network. The level of subscription of a client to multicast sessions may be adapted according to the level of use of the communication network.
Owner:CANON KK
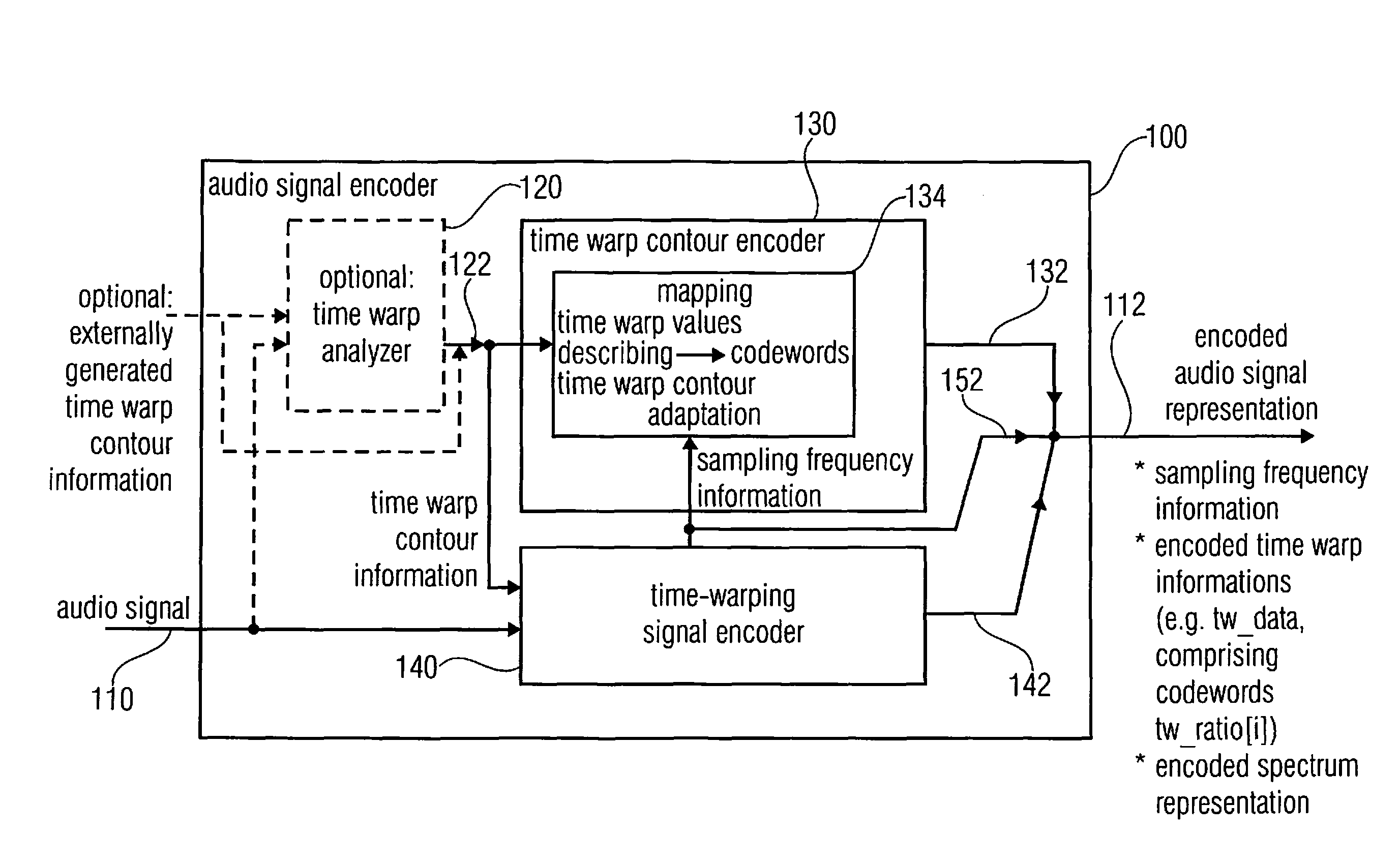
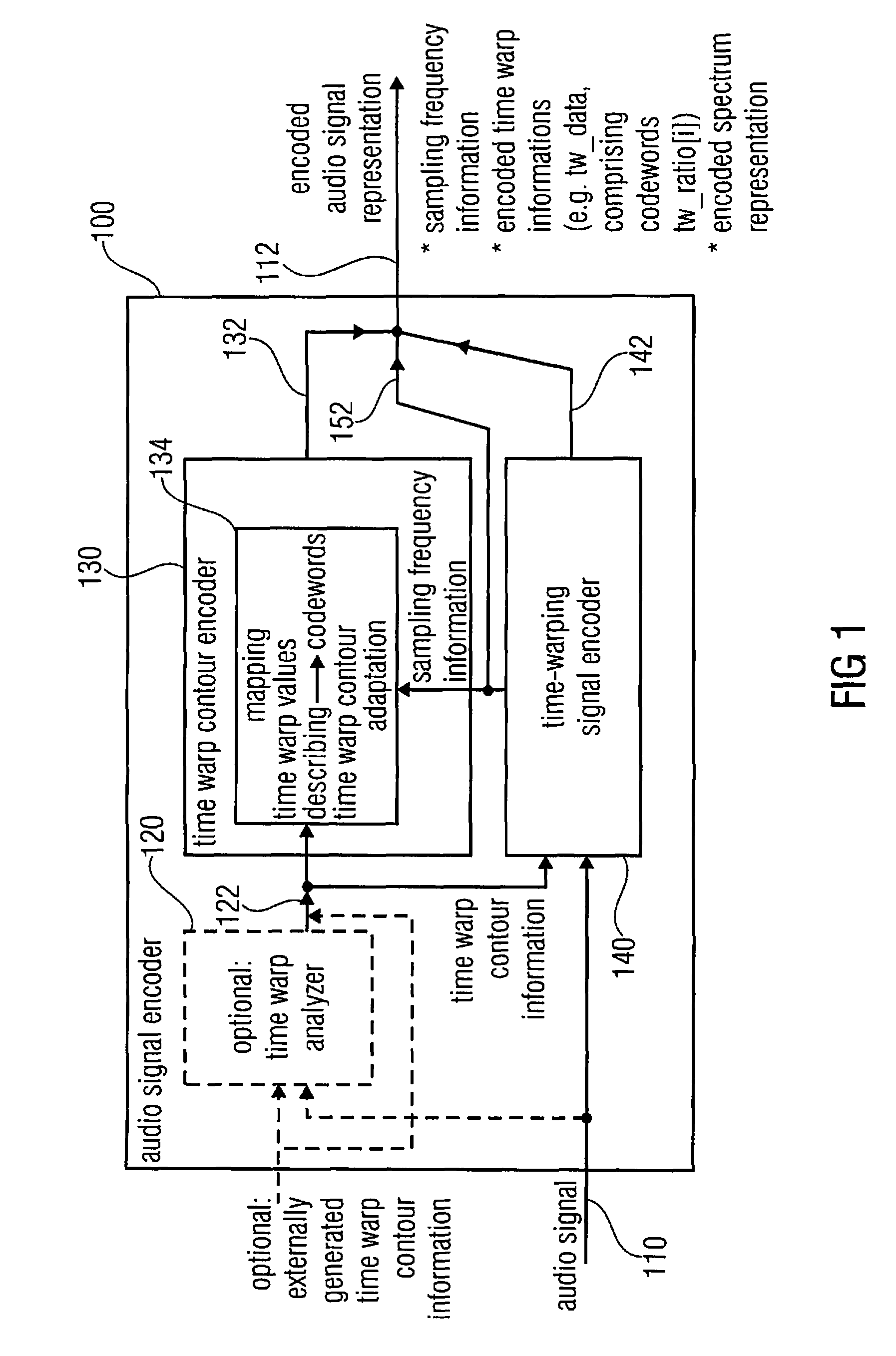
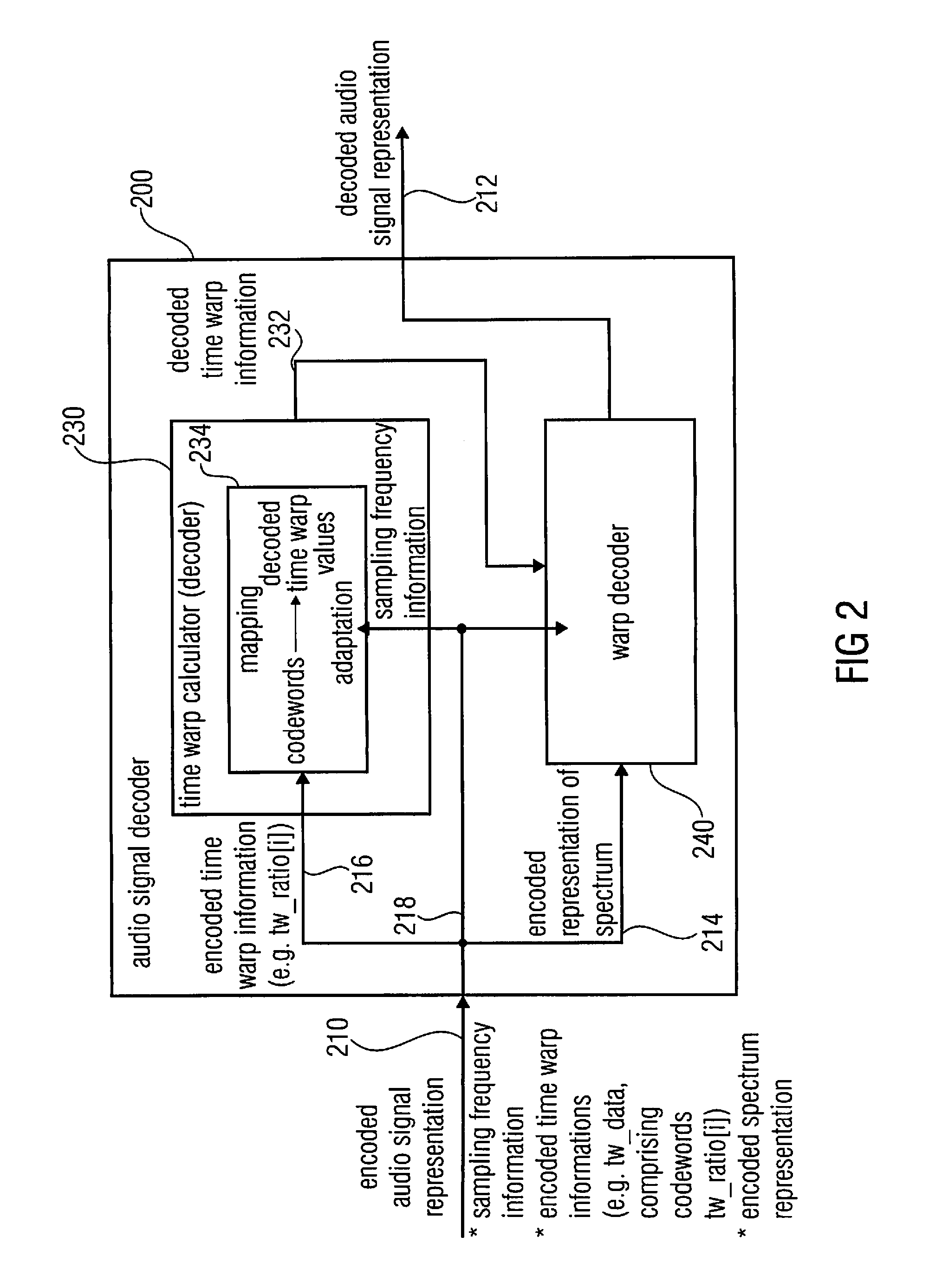
Audio signal decoder, audio signal encoder, methods and computer program using a sampling rate dependent time-warp contour encoding
An audio signal decoder configured to provide a decoded audio signal representation on the basis of an encoded audio signal representation including a sampling frequency information, an encoded time warp information and an encoded spectrum representation includes a time warp calculator and a warp decoder. The time warp calculator is configured to adapt a mapping rule for mapping codewords of the encoded time warp information onto decoded time warp values describing the decoded time warp information in dependence on the sampling frequency information. The warp decoder is configured to provide the decoded audio signal representation on the basis of the encoded spectrum representation and in dependence on the decoded time warp information.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV +1
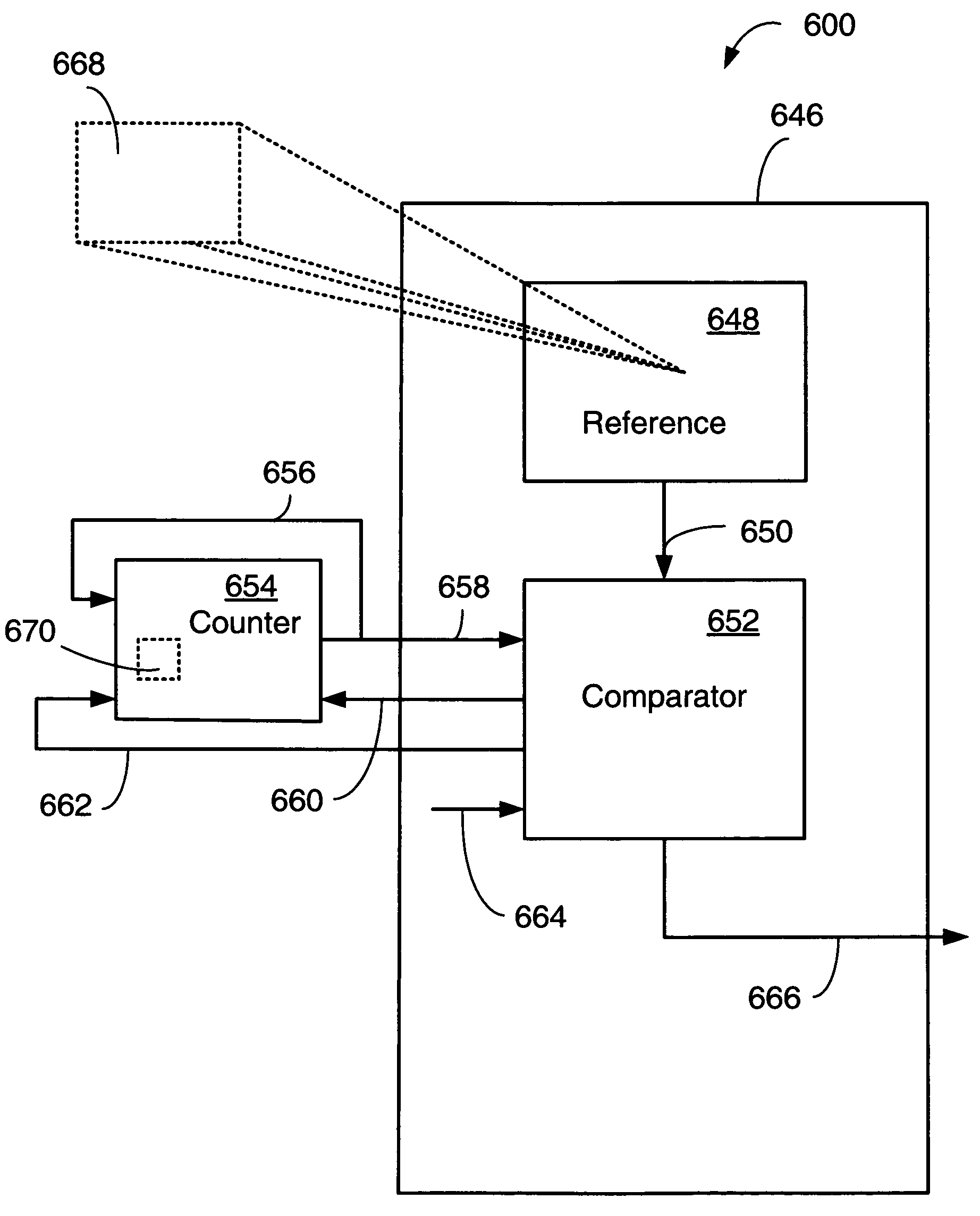
Physically-enforced time-limited cores and method of operation
ActiveUS7183799B1Configure resourceSolid-state devicesLogic circuits using elementary logic circuit componentsProgrammable logic deviceComputer science
A programmable logic device may comprise a metric circuit operable to repeatedly perform a function and emit a first signal dependent upon its advancement into the function. A comparator may compare the first signal from the metric circuit to a predetermined reference signal. A controller may then selectively disable a portion of the programmable logic device dependent upon the results of the comparison. In a particular case, the weakened circuit may be a counter that repeatedly advances its count with a rate dependent upon an aging characteristic of a vulnerable element.
Owner:XILINX INC
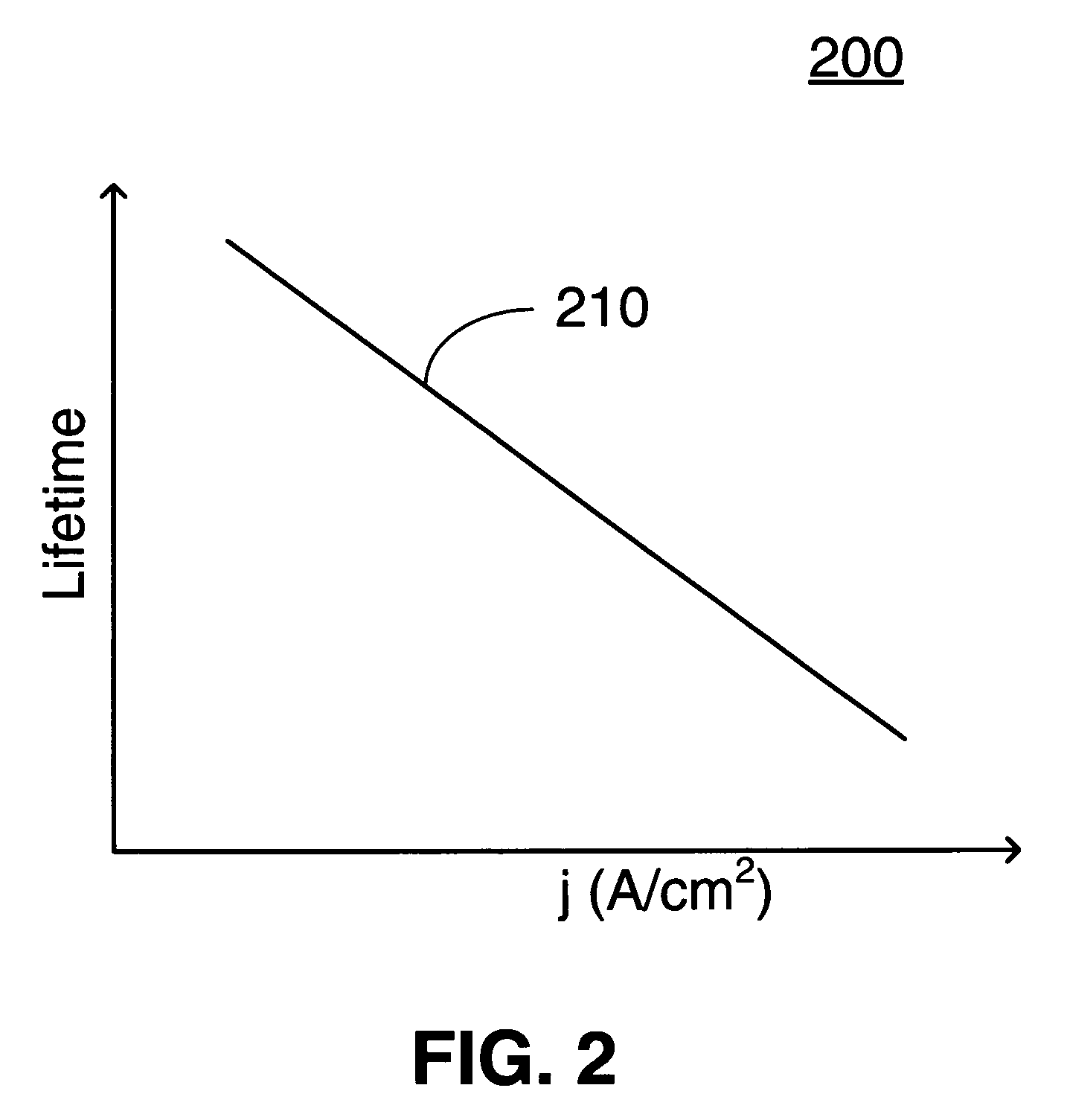
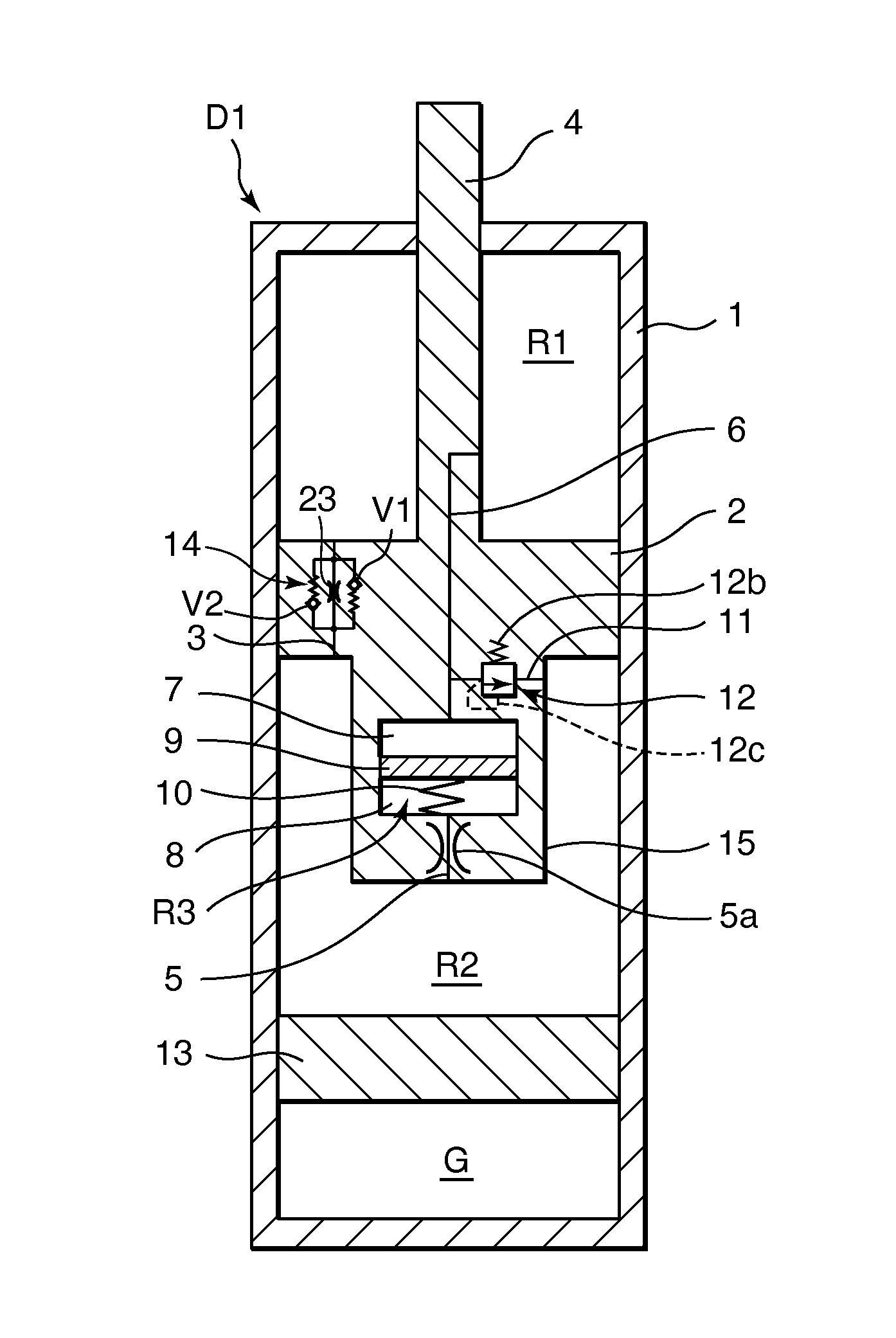
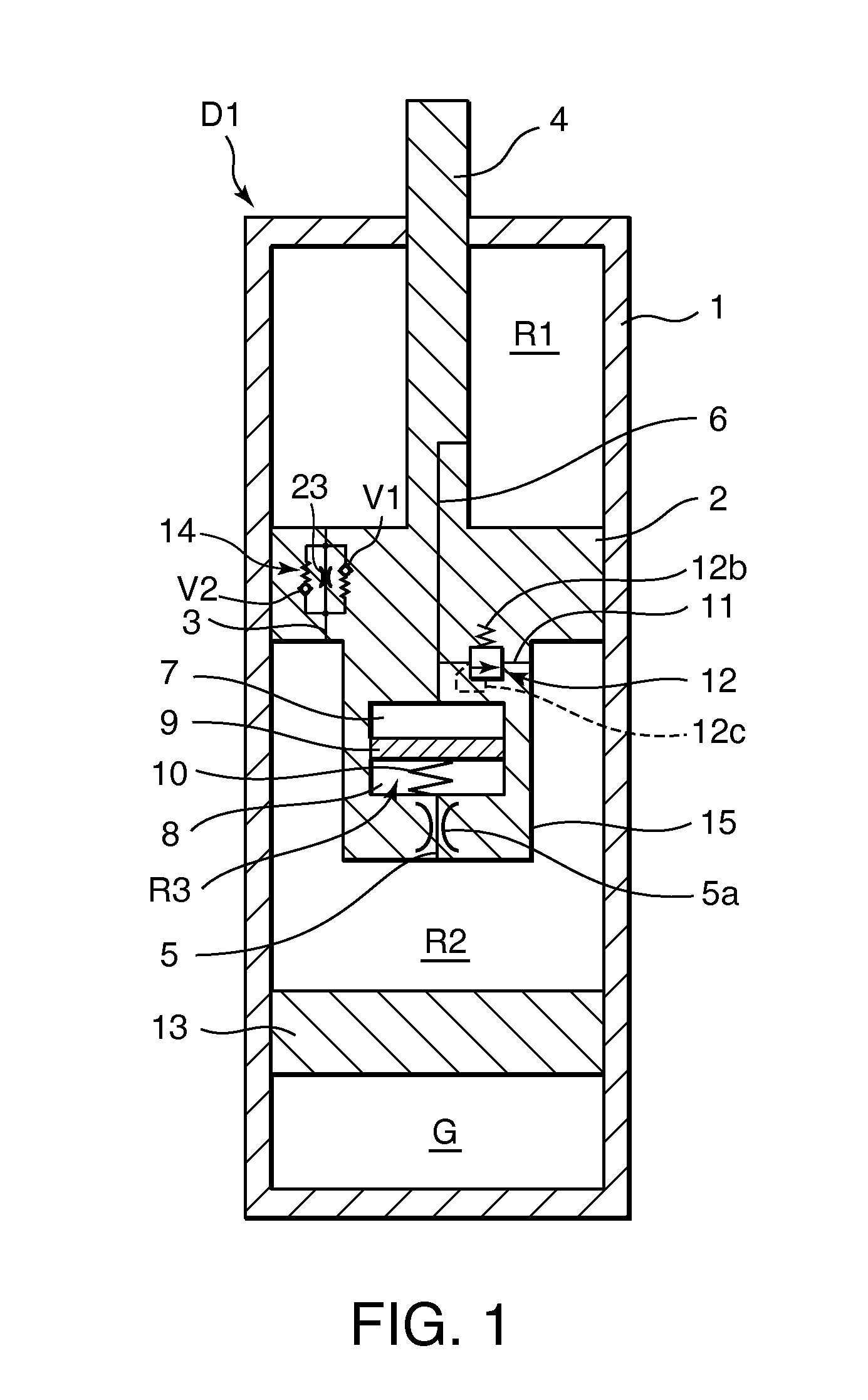
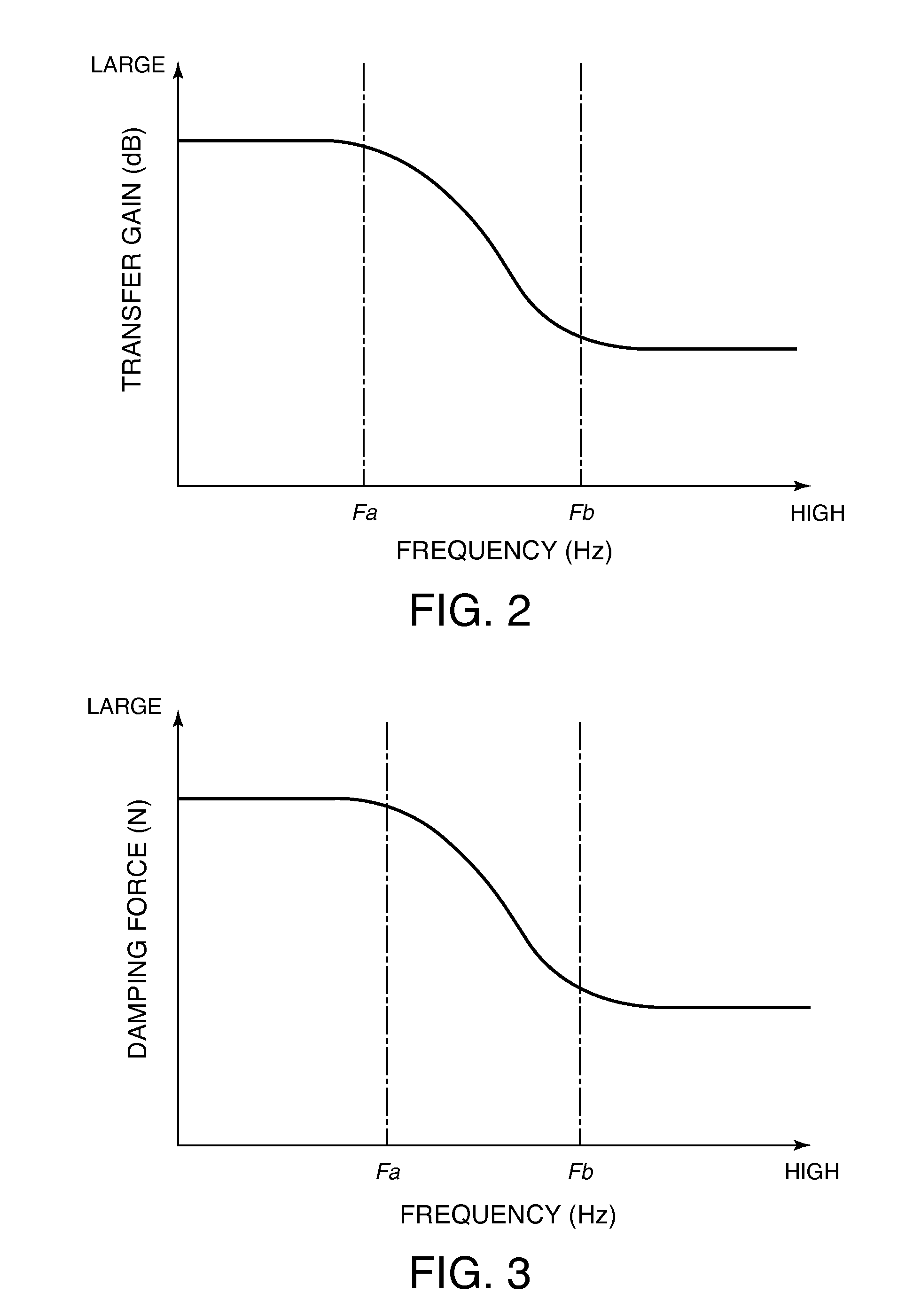
Damping device
ActiveUS20120234639A1Increase flow resistancePassage resistance of to increaseSpringsLiquid based dampersEngineeringRate dependent
A damping device comprises a piston that partitions an interior of a cylinder into first and second working chambers. A flow-rate-dependent damping force generating element connects the first and second working chambers. A first pressure chamber and a second pressure chamber divided by a free piston are formed integrally with the piston. A first connecting passage connects the first working chamber and the first pressure chamber, and a second connecting passage connects the second working chamber and the second pressure chamber. By providing a relief valve that allows fluid to flow from the first working chamber into the second working chamber, an increase in the generated damping force during a high speed operation of the piston can be suppressed, regardless of a vibration frequency of the piston.
Owner:KYB CORP
Football helmet liner to reduce concussions and traumatic brain injuries
A composite multi-axial impact protection liner for a helmet is provided that reduces rotational acceleration, rotational strain rate, and rotational strain that cause concussions. In a protective helmet so equipped, one or more layers of fluid polymer, including strain thinning and strain thickening polymers, are positioned between the wearer's head and a hard helmet shell. The liner offers greater injury protection, performance, and personal comfort using the rate dependent and combined effect of strain thinning and strain thickening of fluid polymer layers.
Owner:ZYMPLR LC
Deposition apparatus for temperature sensitive materials
InactiveUS20090081365A1Low costImproved deposition rate controlVacuum evaporation coatingSputtering coatingMetallurgyVaporization
A system for the deposition of vaporized materials on a substrate is described, comprising at least first and second orientation-independent apparatuses for directing vaporized organic materials onto a substrate surface to form first and second films, each of the first and second orientation-independent apparatuses being arranged in a different relative orientation and comprising: a chamber containing a quantity of material; a permeable member at one end of the chamber with a heating element for vaporizing the material; and means for continuously feeding the material toward the permeable member as it is vaporized, whereby organic material vaporizes at a desired rate-dependent vaporization temperature at the one end of the chamber. A plurality of thin films may be deposited on a substrate using deposition apparatus in a variety of orientations. Such a design provides reduced costs and improved deposition rate control.
Owner:GLOBAL OLED TECH
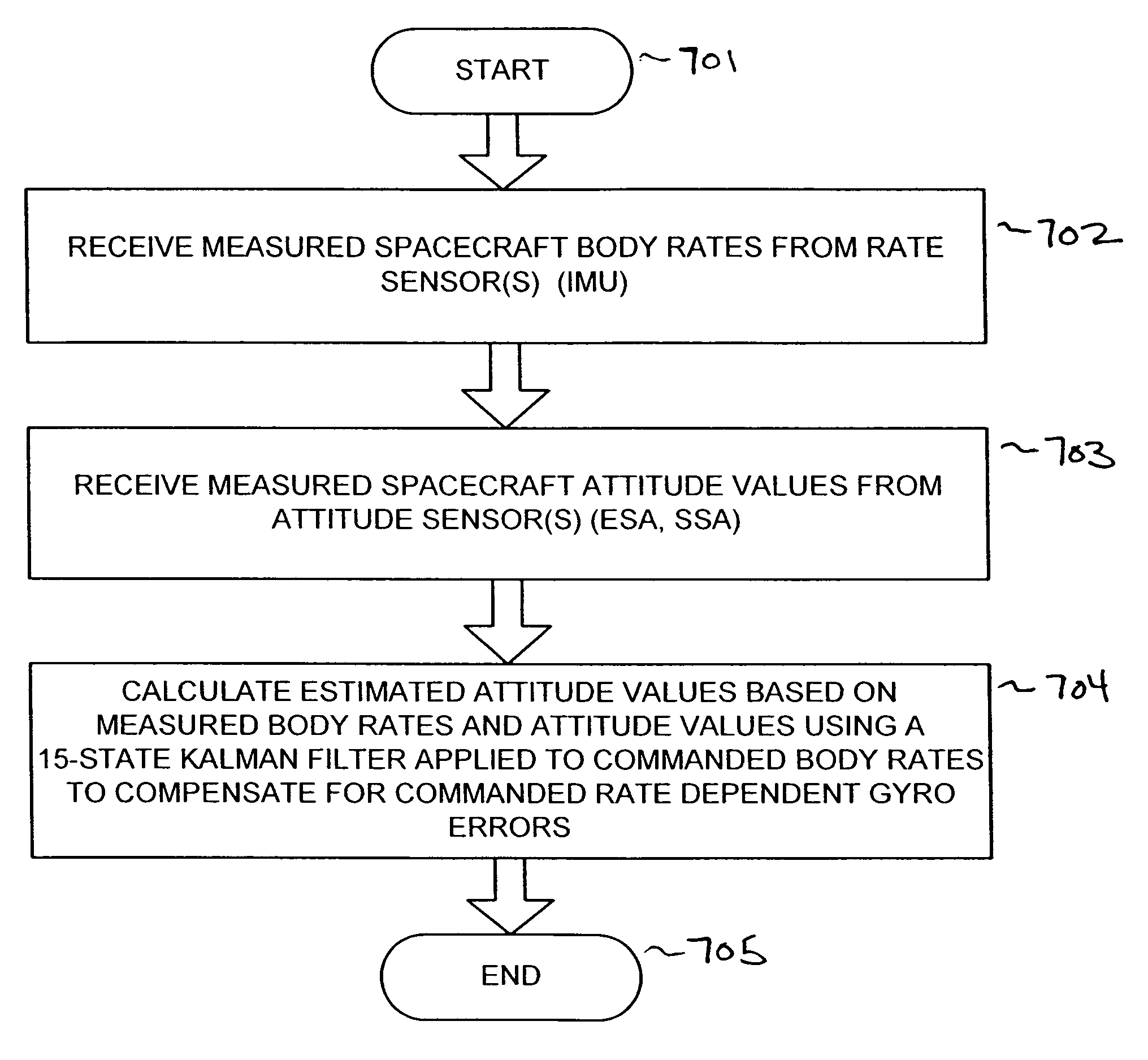
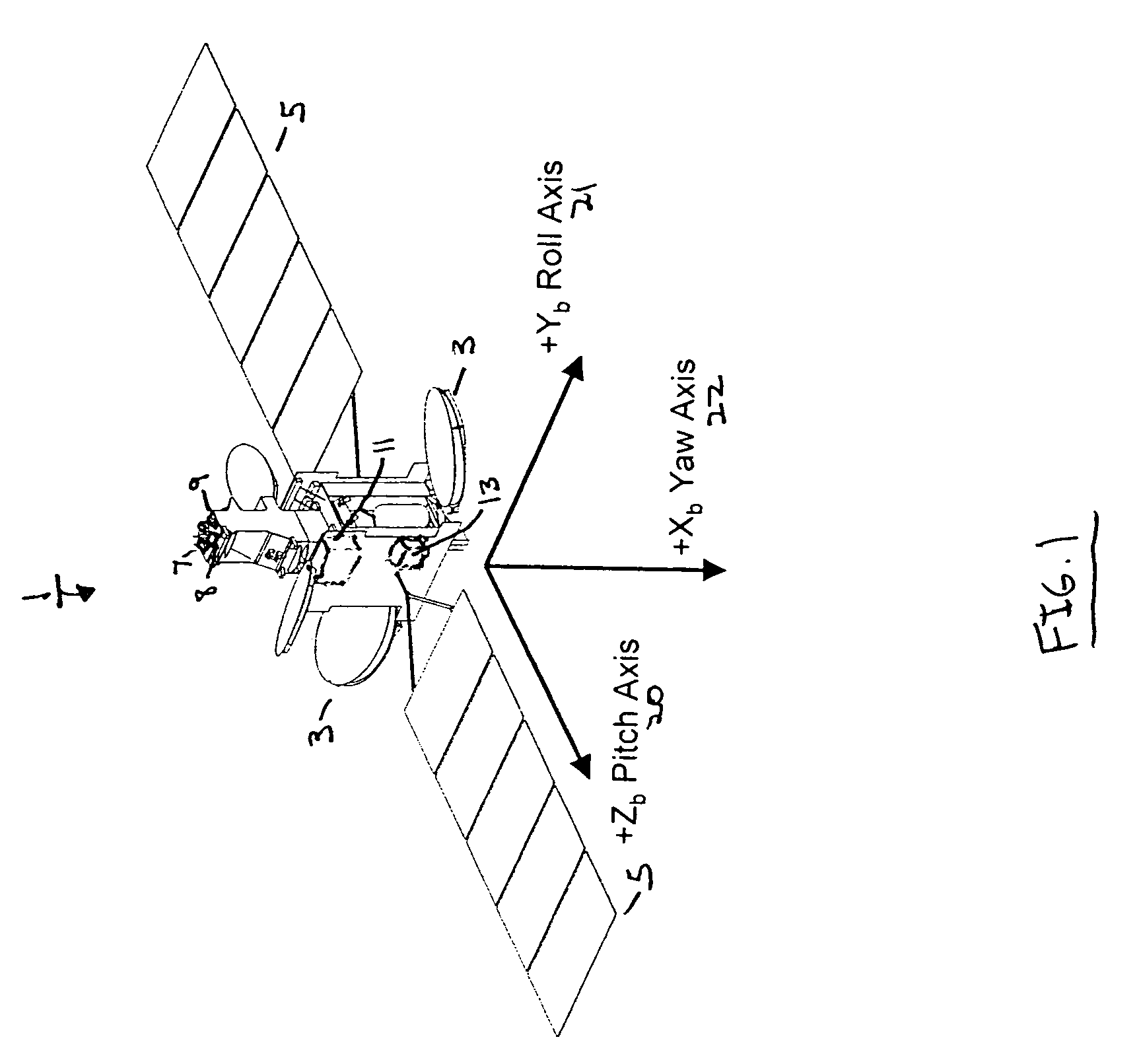
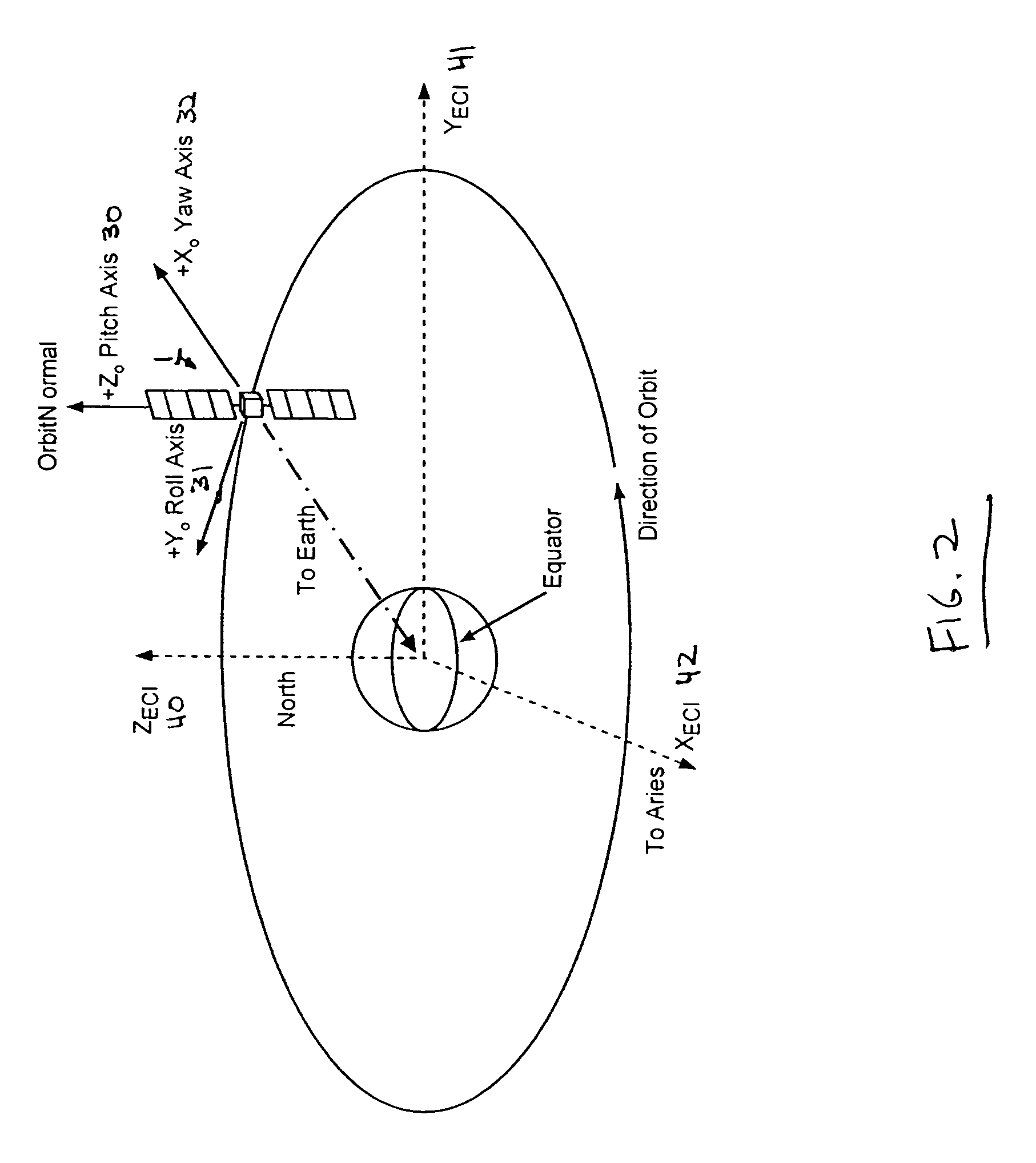
Attitude determination system for yaw-steering spacecraft
InactiveUS7835826B1Accurate valuePointing accuratelyCosmonautic vehiclesDigital data processing detailsKaiman filterGyroscope
An attitude determination system is provided for determining attitude values of a yaw-steering spacecraft, and includes a rate sensor, at least one attitude sensor, and a processor operable to receive measured spacecraft body rates from the rate sensor, to receive measured spacecraft attitude values from the at least one attitude sensor, and to calculate estimated spacecraft attitude values based on the measured spacecraft body rates and the measured spacecraft attitude values by using a Kalman filter that includes a plurality of attitude estimate error states, a plurality of gyro bias states, and a plurality of commanded rate dependent gyro error states.
Owner:LOCKHEED MARTIN CORP
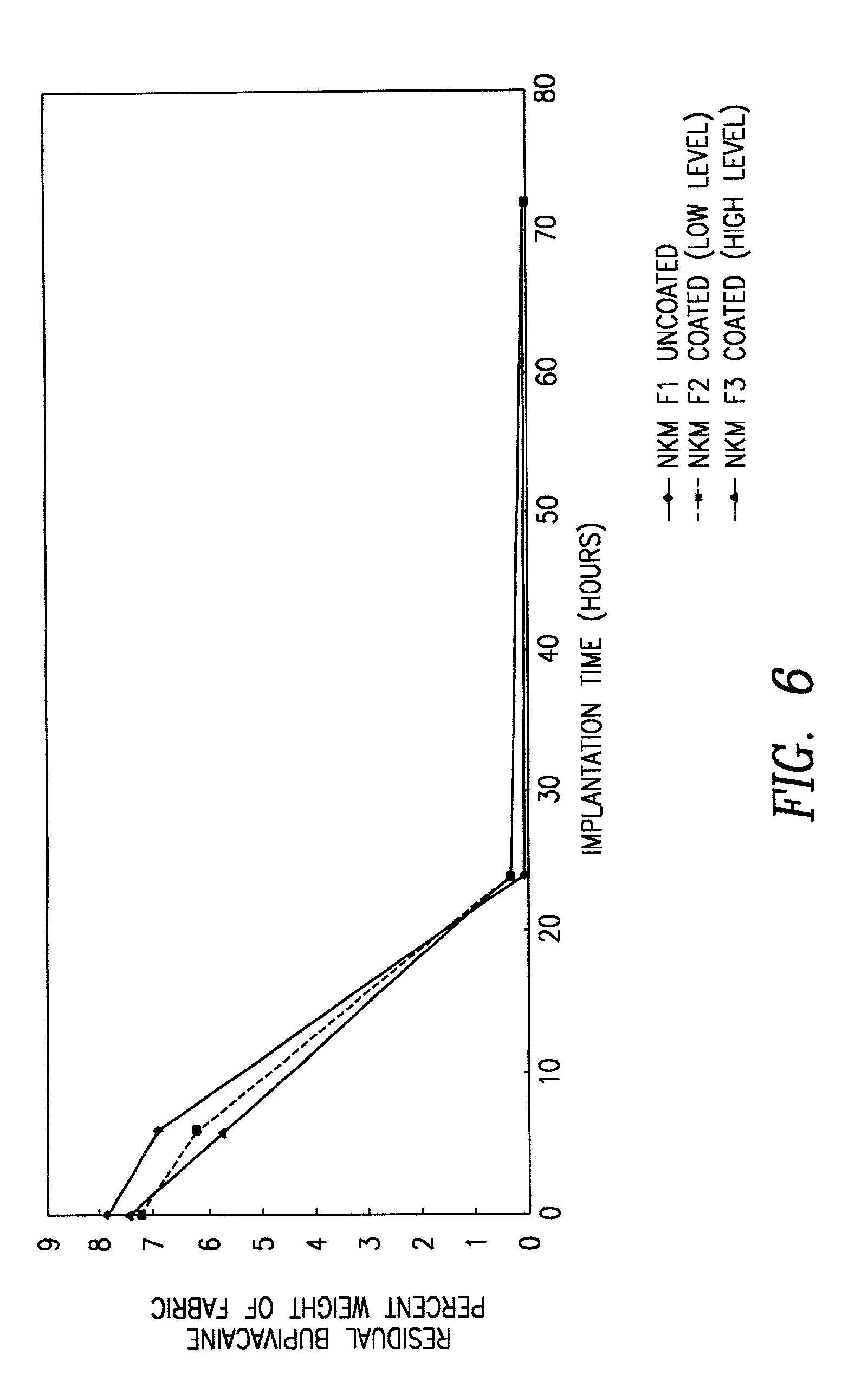
Bio-compatible means for controlled drug delivery to tissue and method of use
There are provided bio-compatible means for delivery of at least one pharmaceutically active agent to a patient in need of same, comprising a bio-compatible, bio-degradable anionic or cationic carrier and at least one pharmaceutically active agent wherein the agent is cationic when the carrier is anionic and is anionic when the carrier is cationic. The delivery means further comprises at least one bio-compatible enclosing means for the carrier. This enclosing means may be bio-degradable or non bio-degradable and have a predetermined permeation gradient for the passage therethrough of the at least one pharmaceutically active agent. In an alternate embodiment the enclosing means may be biodegradable without a predetermined permeation gradient for the passage therethrough of the at least one pharmaceutically active agent. In all embodiments the active agent is ionically linked to the carrier and the carrier / active agent combination is enclosed in the enclosing means. There is also provided a method of administering at least one pharmaceutically active agent to the tissue surface of a subject in need of same at a rate dependent on the permeability and / or bio-degradability of the enclosing means of the delivery means which comprises contacting the tissue surface with a bio-compatible delivery means as described above.
Owner:ETHICON INC
Direct metering fuel system with an integral redundant motor pump
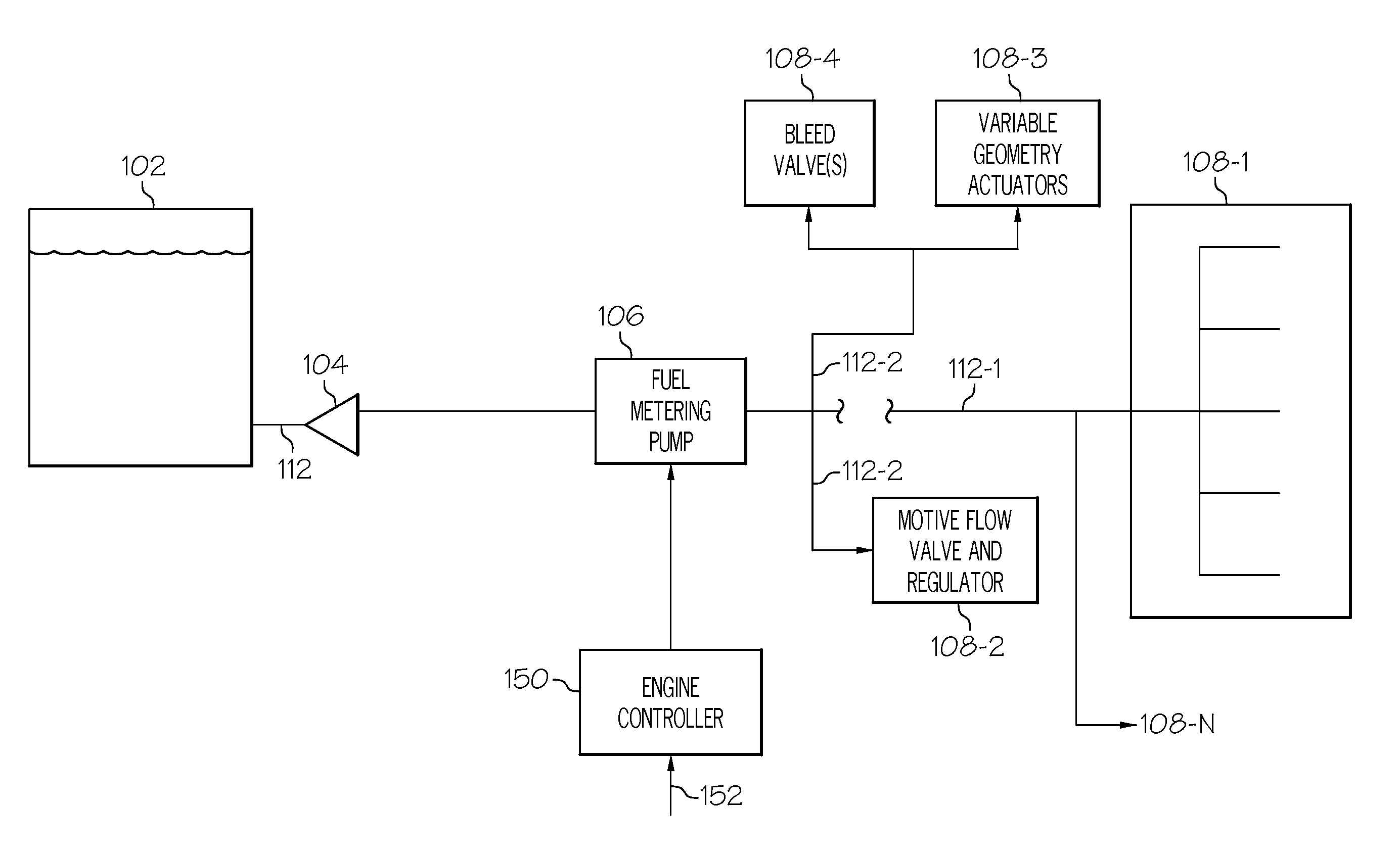
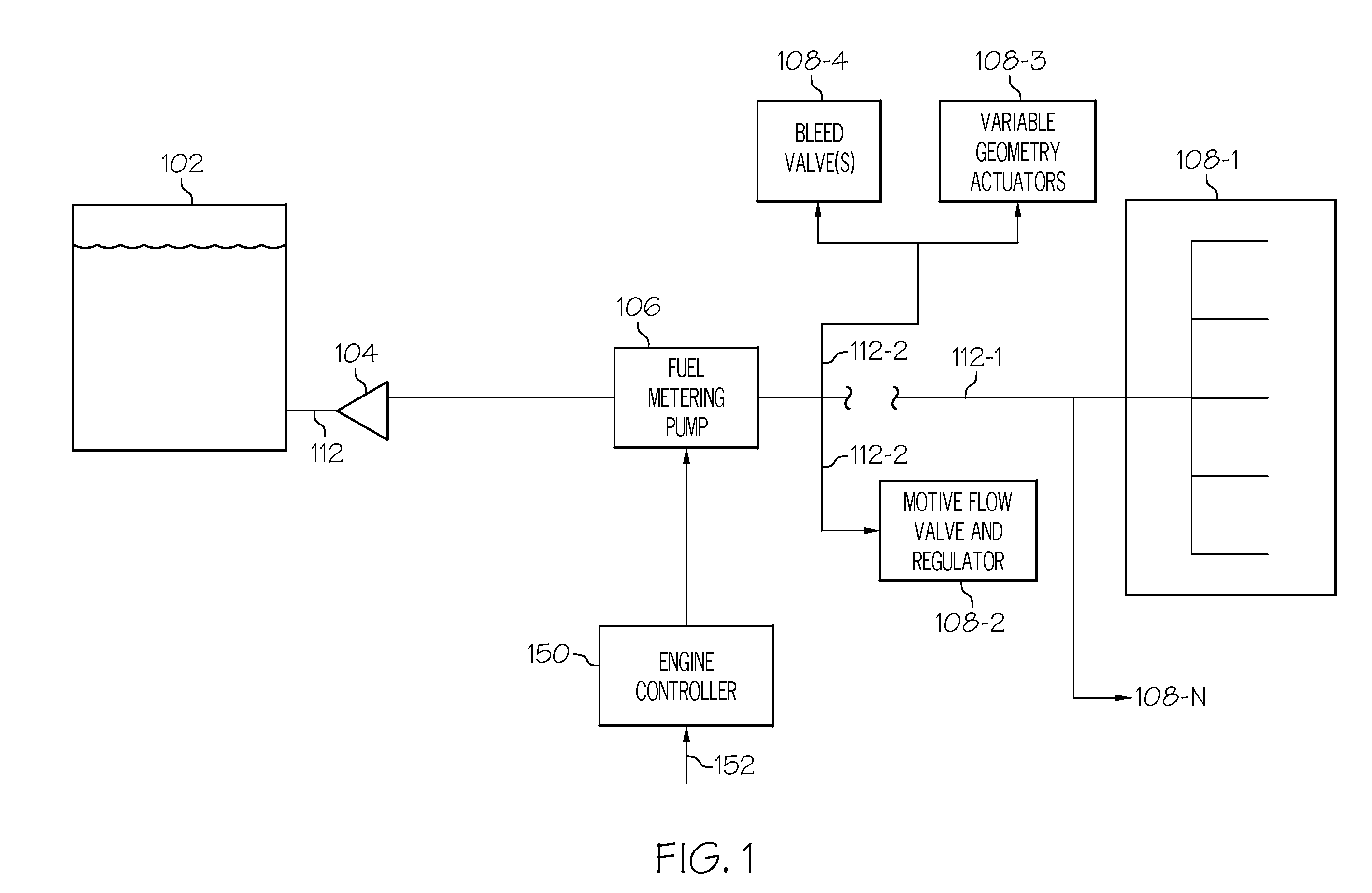
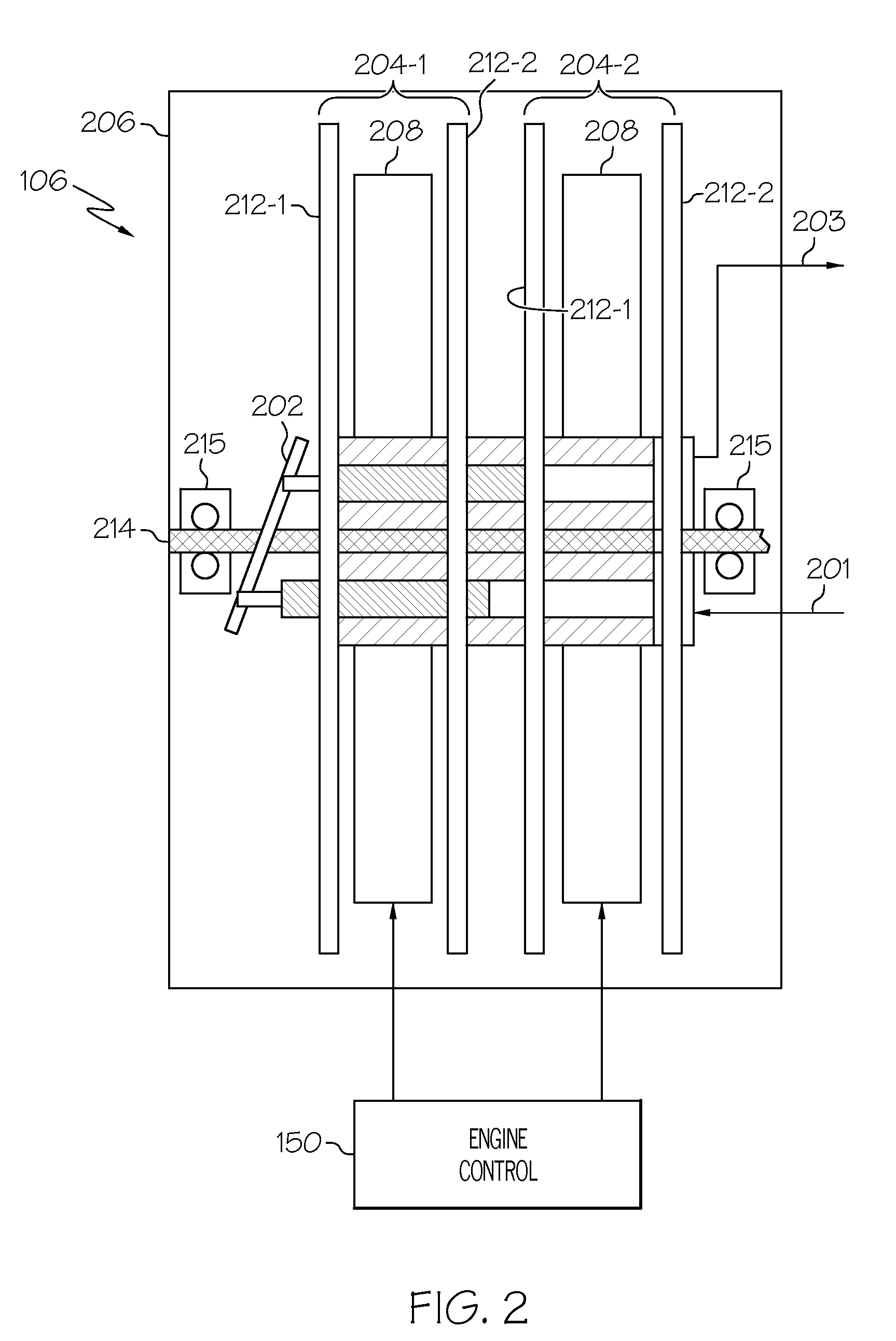
ActiveUS20090071442A1Liquid fuel feeder/distributionPositive displacement pump componentsControl theoryTurbine
A direct metering fuel supply system includes a plurality of axial gap motors, a fixed displacement, variable speed positive displacement piston pump, and a gas turbine engine control. Each axial gap motor is configured to be selectively energized and is operable, upon being energized, to supply a drive torque at a drive speed. The fixed displacement, variable speed positive displacement piston pump is coupled to each of the axial gap motors to receive the drive torque selectively supplied therefrom and is operable, upon receipt of the drive torque, to supply fuel at a flow rate dependent on the drive speed. The gas turbine engine control is adapted to receive fuel flow commands and is operable, in response to the fuel flow commands, to energize one of the plurality of axial gap motors.
Owner:HONEYWELL INT INC
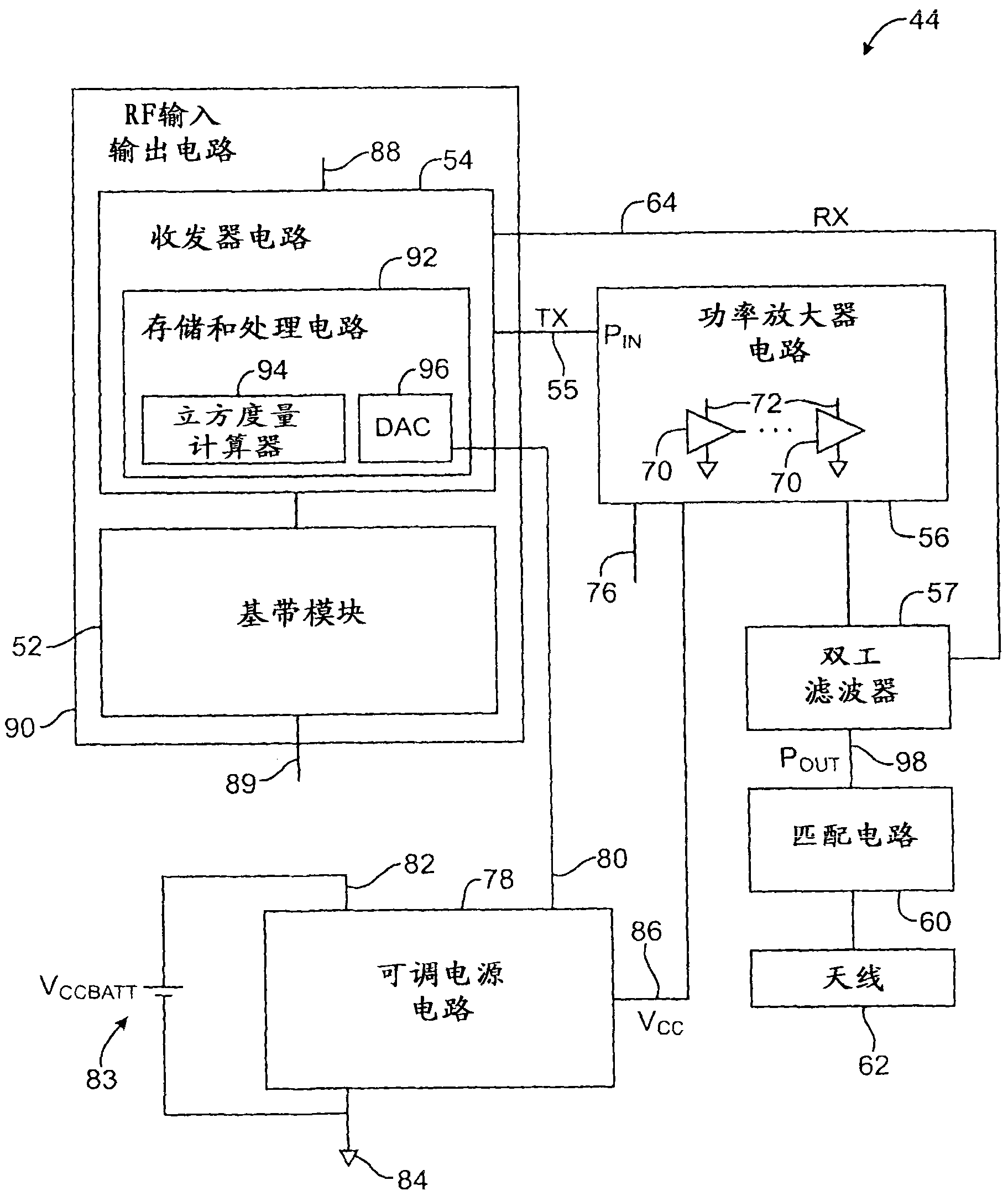
Electronic device with data-rate-dependent power amplifier bias
ActiveCN101888263AAdd other featuresEnergy efficient ICTPower managementAudio power amplifierRadio frequency signal
Wireless circuitry in an electronic device may contain output power amplifier circuitry for amplifying transmitted radio-frequency signals. The power amplifier circuitry may be powered using a bias voltage. The magnitude of the bias voltage can be selectively reduced to conserve power. Control circuitry can maintain a table of bias voltage settings to use under various conditions. These conditions may include required output powers as determined by link quality, transmission mode status, and required data rates. When link quality is low or when high data rates are required, the bias voltage can be maintained at a relatively high level to ensure that the power amplifier operates linearly and does not exhibit excessive noise. When link quality is high or when data rates are low as with voice calls, the bias voltage can be reduced to conserve power.
Owner:APPLE INC
Circulation pump, heating system and method of determining the flow rate of a liquid through a pipe
InactiveUS20090121034A1Save on installation costsGuaranteed uptimePump componentsLighting and heating apparatusImpellerSwitching signal
In order to provide a circulation pump for a conveyed liquid, comprising an electric motor, which is electronically commutated and has a rotor, a stator and a motor circuit, and an impeller that is connected in a rotationally fixed manner to the rotor, with advantageous properties, it is provided that the electric motor has an evaluation device, by means of which a flow rate of conveyed liquid through the circulation pump can be determined from a rotational speed of the rotor and / or a power consumption of the electric motor, and at least one signal output is provided, at which a flow rate signal and / or flow rate-dependent switching signal can be supplied by the circulation pump.
Owner:ITT MFG ENTERPRISES LLC
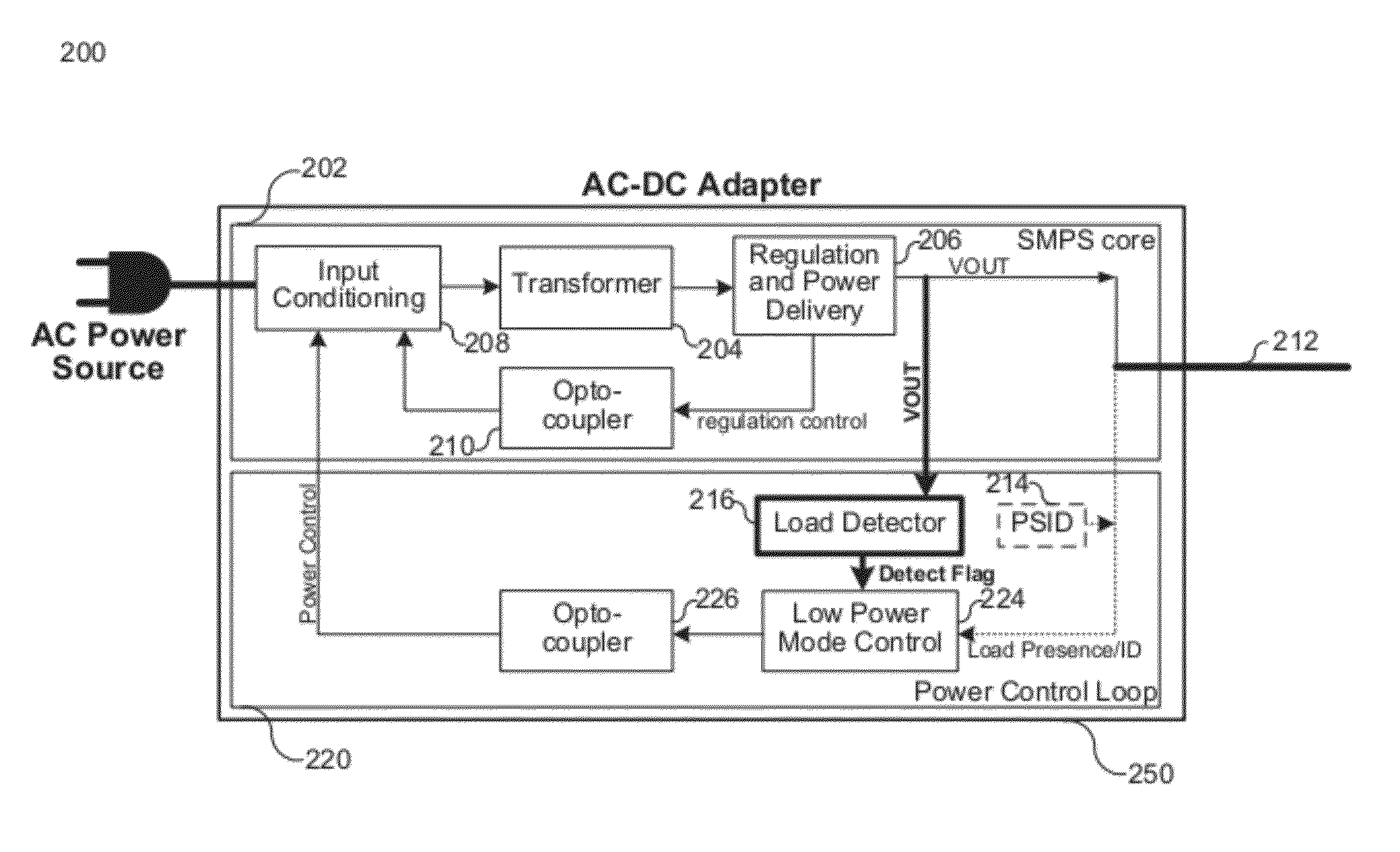
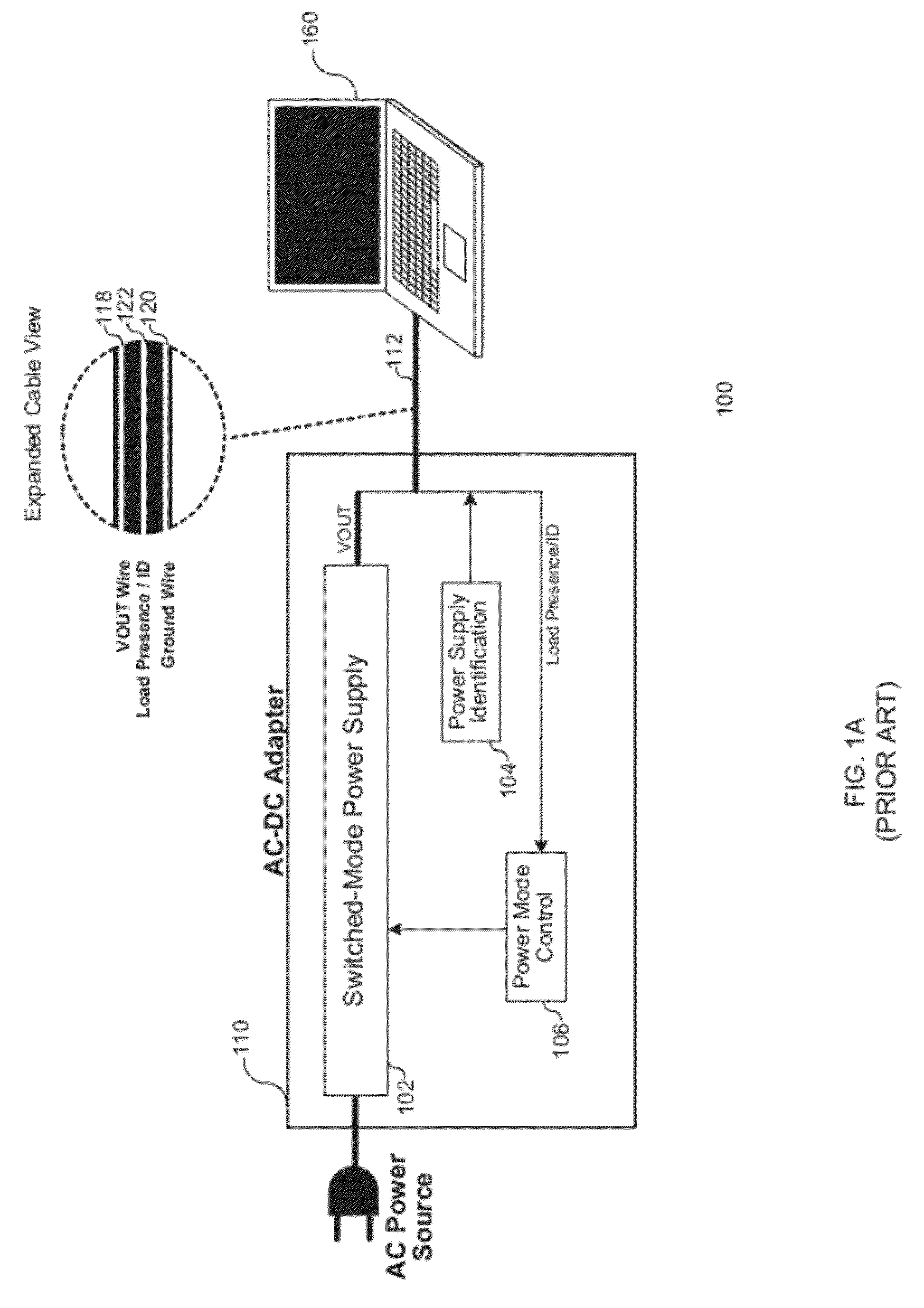
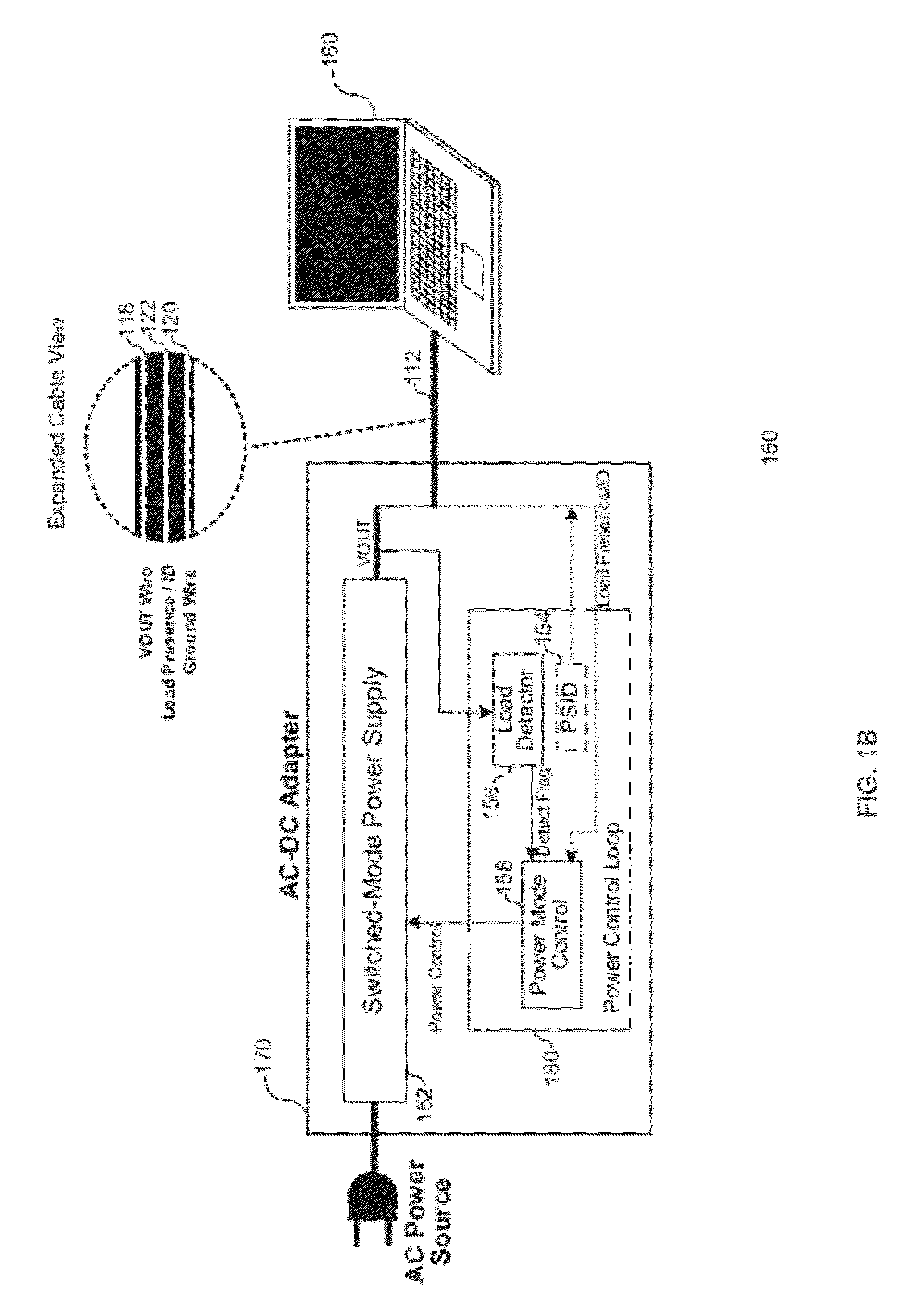
Load Detection for a Low Power Mode in an AC-DC Adapter
ActiveUS20120206947A1Ac-dc conversion without reversalEfficient power electronics conversionPower modeElectricity
Various embodiments of the present invention relate to AC-DC adapters, and more particularly, to systems, devices and methods of employing a load detector to detect a load condition of the AC-DC adapters based on transient variation of a DC output voltage; and therefore, enable the AC-DC adapters to switch from a low power mode to a normal power mode. At the low power mode, a DC output voltage is generated at a target voltage by the AC-DC adapter, and subsequently drops at a decay rate dependent on load condition. The load detector is electrically coupled to monitor the output voltage and measure the decay time between two threshold voltages. An enable instruction is provided to configure the AC-DC adapter to the normal power mode as soon as the decay time is lower than the threshold decay time or the variation of consecutive decay times reaches a certain threshold.
Owner:MAXIM INTEGRATED PROD INC
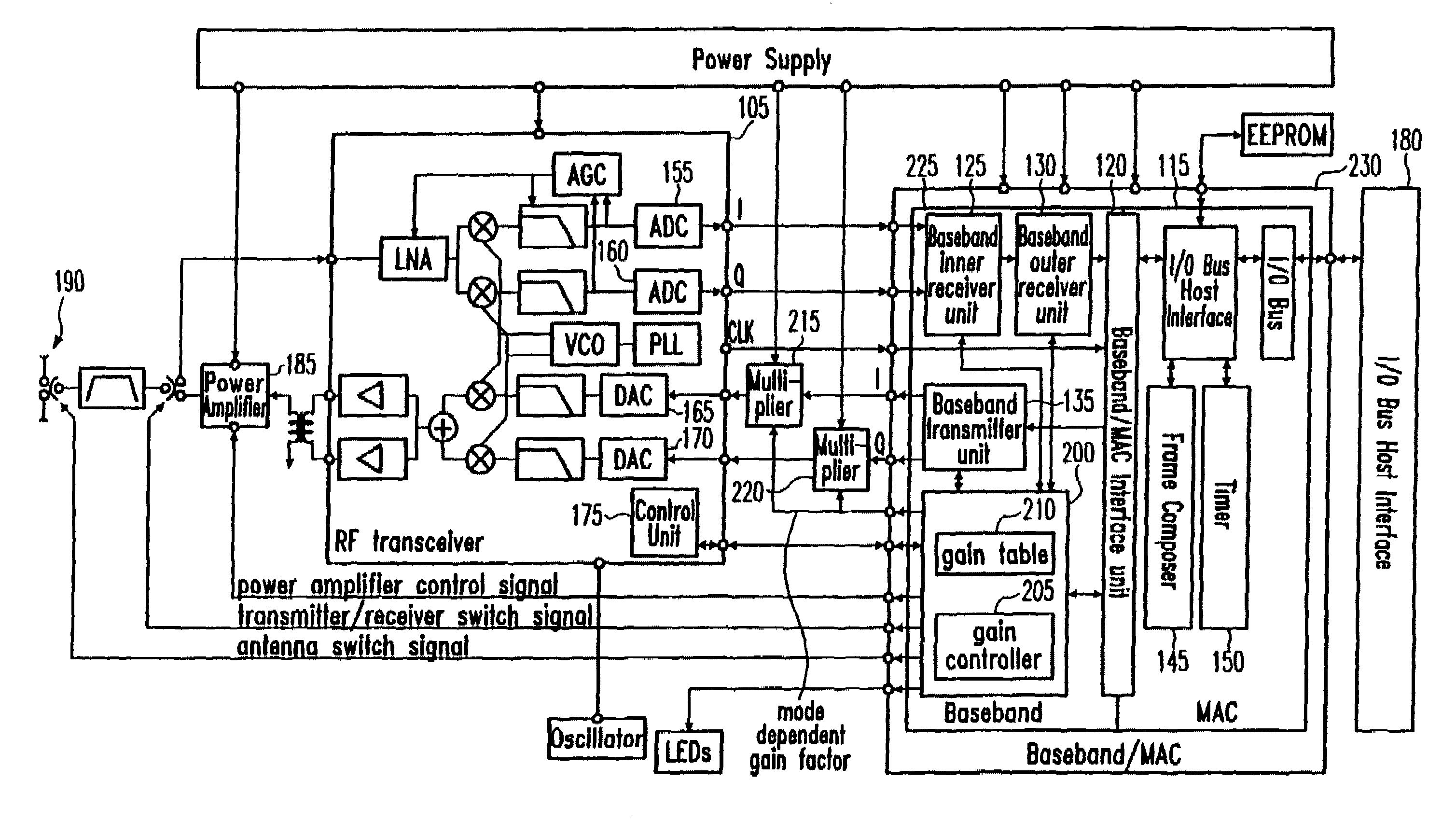
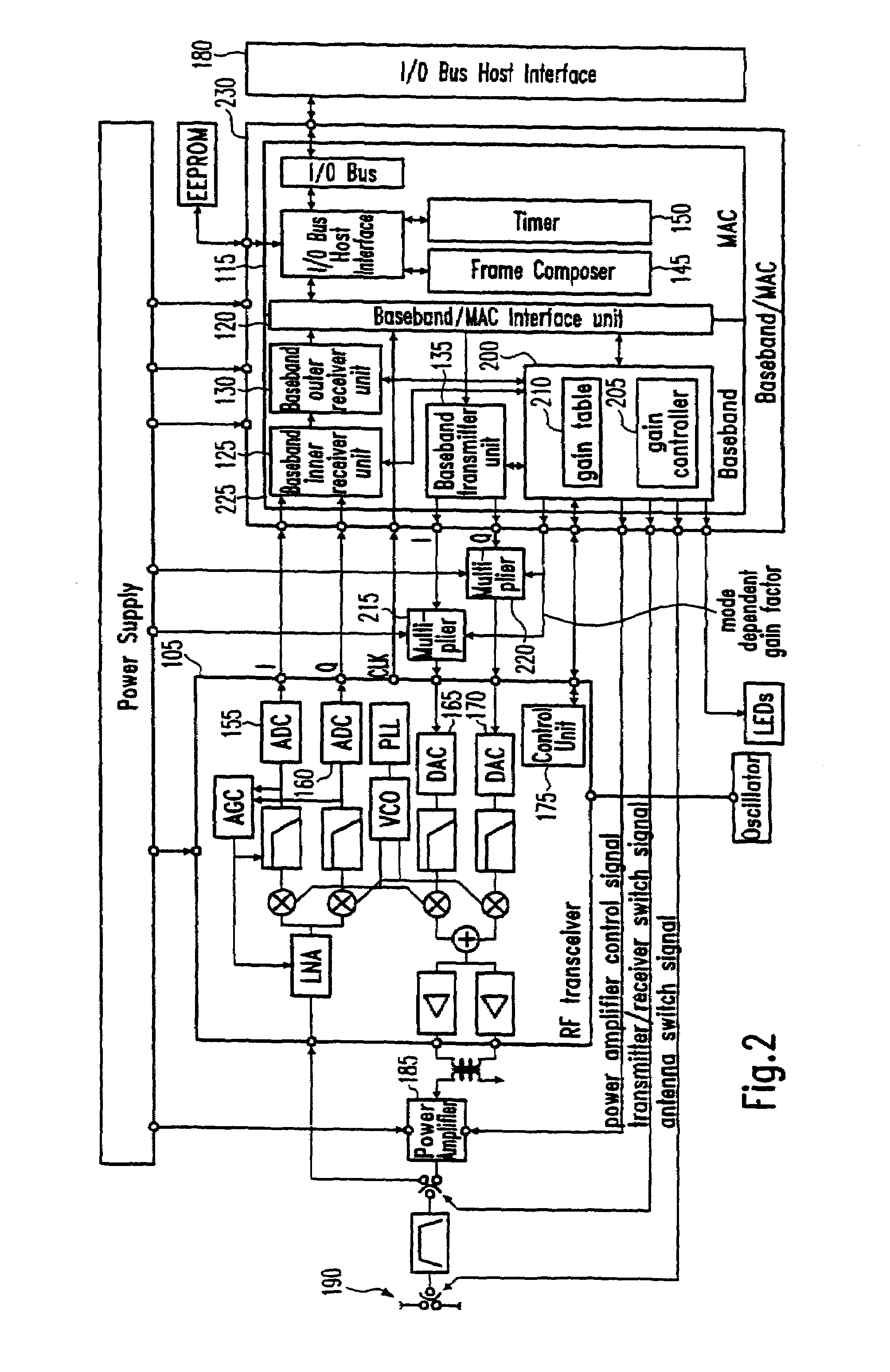
Rate dependent transmission gain control for WLAN systems
A WLAN (Wireless Local Area Network) transmission technique is provided where data is transmitted in two or more different transmission modes at different transmission rates. A transmission gain is determined to be applied when transmitting data. The transmission gain is determined to be transmission mode dependent such that the transmission gain in a first transmission mode is greater than the transmission gain in a second transmission mode if the transmission rate in the first transmission mode is lower than the transmission rate in the second transmission mode.
Owner:ADVANCED MICRO DEVICES INC
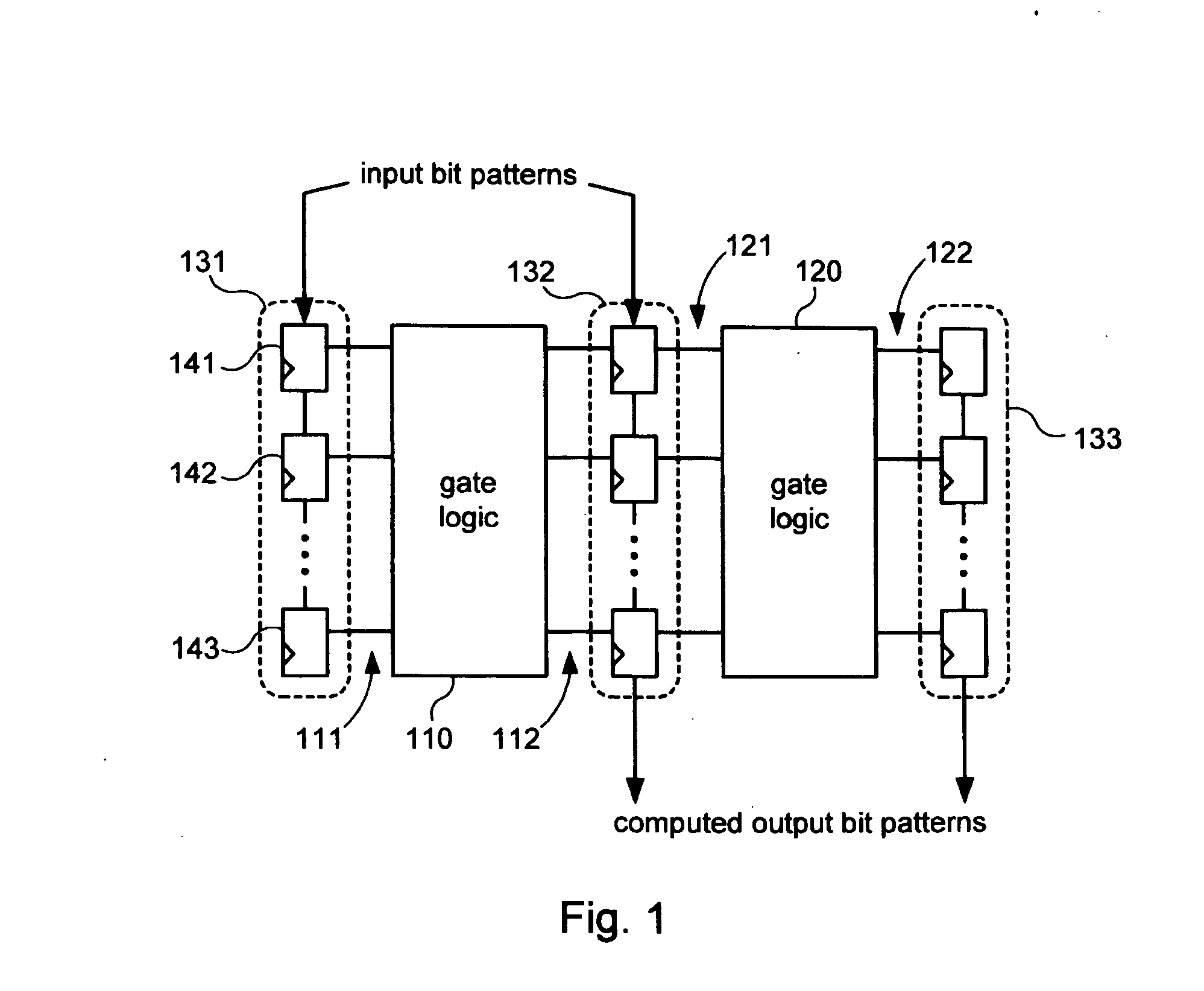
Systems and methods for diagnosing rate dependent errors using LBIST
InactiveUS7475311B2Electronic circuit testingError detection/correctionLogic built-in self-testComputer science
Systems and methods for performing logic built-in self-tests (LBISTs) to detect “at-speed” errors in a digital circuit. In one embodiment, an input bit pattern is propagated through target logic of the digital circuit and captured in scan chains at a normal operating speed to produce a first output bit pattern. This is repeated with the first input bit pattern at a lower test speed to produce a second output bit pattern. Differences between the first and second output bit patterns are then detected to determine whether operation of the digital circuit at the normal operating speed causes errors that are not generated at the lower test speed.
Owner:KK TOSHIBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com