Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain
a glutamic acid decarboxylase and spinal cord injury technology, applied in the direction of lyases, dsdna viruses, peptide sources, etc., can solve the problems of long pain, impede effective rehabilitation, adversely affect the quality of life,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0059]This example demonstrates the construction of the GAD vector.
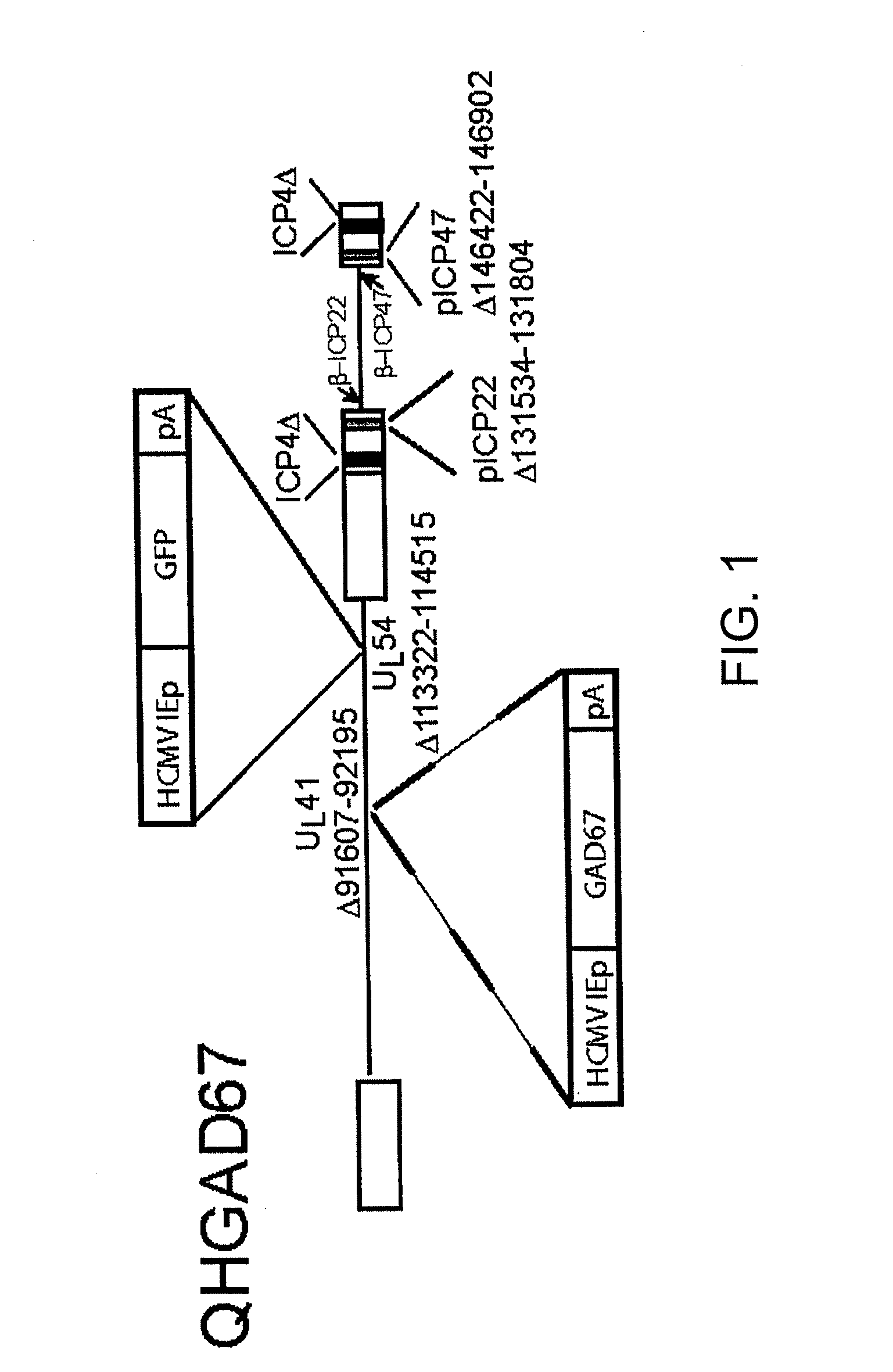
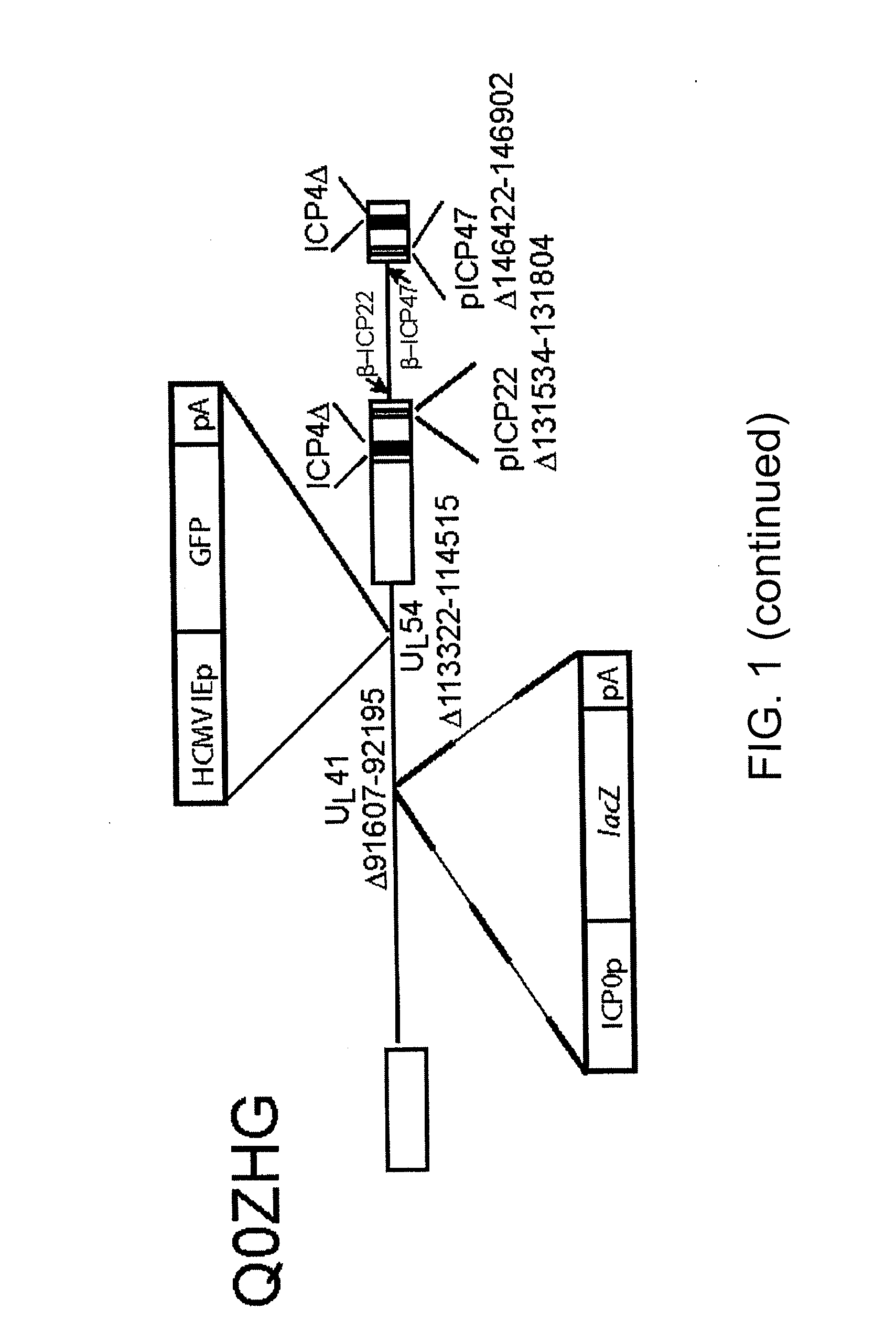
[0060]The nonreplicating HSV vector QHGAD67 is defective in expression of the HSV immediate early (IE) genes ICP4, ICP22, ICP27, and ICP47, and contains the human GAD67 gene under the control of the human cytomegalovirus immediate early promoter (HCMV IEp) in the UL41 locus (FIG. 1). Control vector Q0ZHG (constructed according to the method described in Chen et al. J. Virol, 74(21), 10132-41 (2000)) is defective in the same genes, but contains the Escherichia coli lacZ reporter gene in the same position (FIG. 1).
[0061]GAD67 cDNA (constructed according to the method described in Bu, D. F., et al., Proc. Natl. Acad. Sci. USA, 89, 2115-2119 (1992)) was individually sub-cloned as a ClaI / Xbal fragment downstream of the human cytomegalovirus immediate early promoter in the shuttle plasmid p41H, containing the promoter and adequate HSV flanking DNA sequence in order to enable efficient homologous recombination at the UL41 g...
example 2
[0062]This example demonstrates the construction of an HSV vector having extended deletions of the ICP4 and ICP27 loci and a deletion of UL55.
[0063]The schematic for constructing this vector is set forth in FIG. 14. Specifically, plasmid d106 (Hadjipanayis and DeLuca, Can Res 65(12): 5310-6 (2005)) was virally crossed with plasmid TOZ.1 (Arafat et al., Clin Can Res 6: 4442-8 (2000)) to produce QOZHG.1. The details of vector QOZHG.1 are described in Example 1 and its construction described in Chen et al., J Virol 74(21), 10132-41 (2000).
[0064]Plasmid pPXE (Niranjan et al., Mol Ther 8(4):530-42 (2003)) was recombined into the ICP27 locus of QOZHG.1 to rescue the previous ICP27 deletion and to remove HCMV-eGFP gene. A single recombinant was then isolated, purified and verified by selecting a plaque that did not exhibit green fluorescence under a fluorescent microscope. The recombinant was termed E1. E1 was negative for the GFP gene and positive for the LacZ gene.
[0065]Plasmid 41HN was ...
example 3
[0079]This example demonstrates the expression of GAD protein by GAD vector transduced cells.
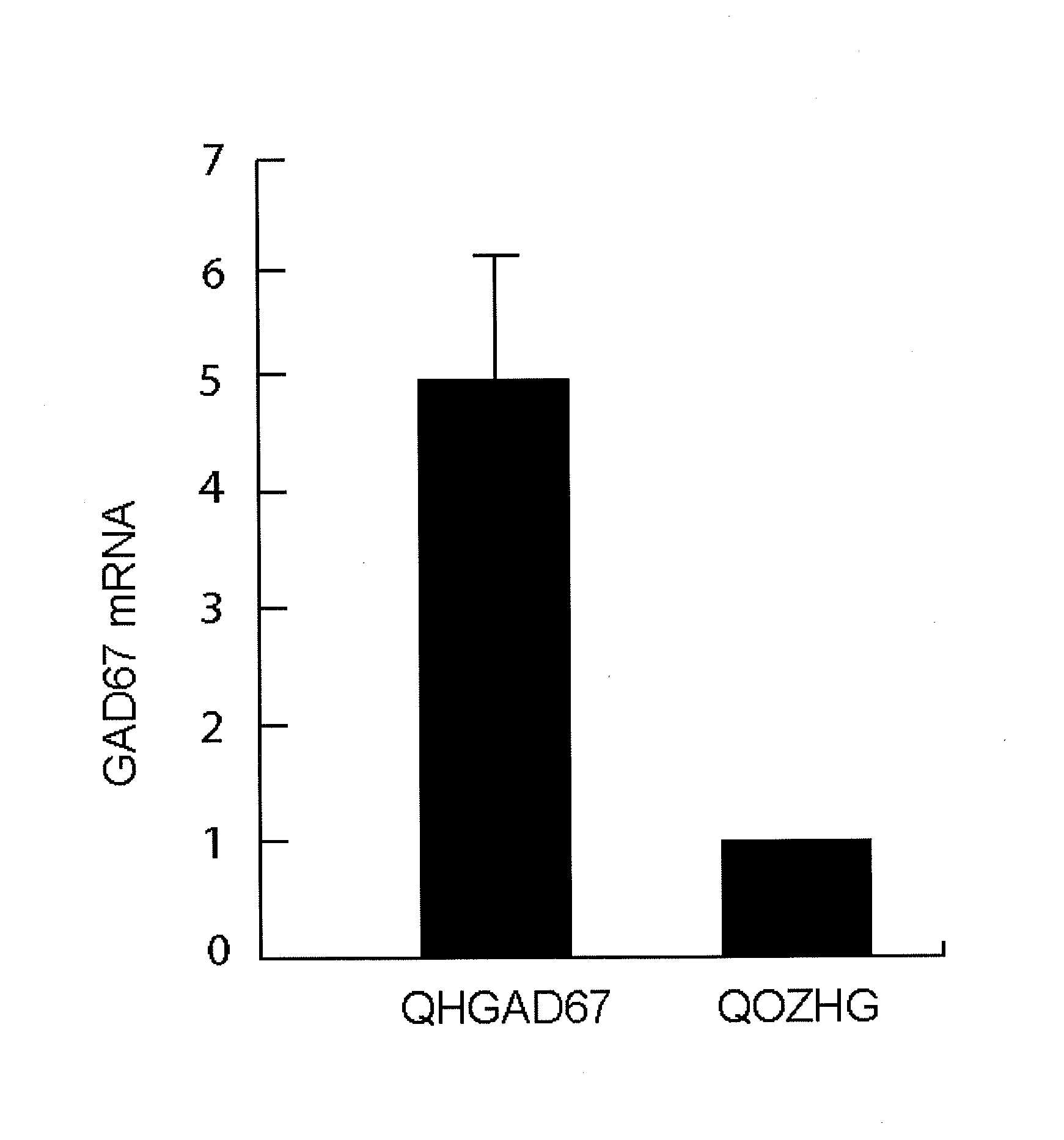
[0080]One week after subcutaneous inoculation of QHGAD67 into the plantar surface of the hind paw of a laboratory rat the amount of GAD67 mRNA in the pooled L4-L6 DRG detected by real-time RT-PCR was fivefold greater than in contralateral DRG transduced with Q0ZHG (FIG. 2). Also, GAD67 immunoreactivity in transduced DRG was present in neurons in a broad spectrum of DRG neurons of all sizes compared to the contralateral (vehicle-injected) DRG. GAD67 protein, determined by Western blot, was significantly increased in both the lumbar DRG (0.048±0.009 OD units) compared to sham-inoculated controls (0.025±0.006 OD units, P<0.01.
[0081]One week after subcutaneous inoculation of 30 μl of 11×109 pfu / ml QHGAD67 increased immunoreactivity was seen predominantly in laminae II and III compared to the contralateral (vehicle-injected) dorsal horn. In the superficial dorsal horn of control rats GAD67 immuno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative optical density | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com