Process for t cell expansion
a technology process, which is applied in the field of t cell expansion, can solve the problems that the number of donor immune cells necessary to effect immune reconstitution against a specific pathogen cannot be obtained through simple mechanical selection systems, and many of these may not be functioning or may be functioning sub-optimally, so as to reduce the time and resources required, and minimise the risk of contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Process for 226 CMV
[0214]Day −0
[0215]Buffer Preparation
[0216]IL-4
[0217]A 50 μg vial of IL-4 (USP grade, CellGenix cat 1003-050) was diluted with 2504 of WFI (USP Grade Invitrogen Cat A12873) to produce a 200 μg / ml stock solution. The stock solution was stored at −80° C. with an aliquot being left for use in the pot inoculation
[0218]IL-7
[0219]A 50 μg vial of IL-4 (GMP, Cellgenix cat 1010-050) was diluted with 200 μl of WFI (USP Grade Invitrogen Cat A12873) to produce a stock. The stock was diluted 1:25 with WFI to produce a working stock, which was then stored at −80° C. with an aliquot being left for use in the pot inoculation.
[0220]CMV PepTivator Peptide
[0221]A 60 nmol / peptide peptide (GMP PepTivator pp65) was reconstituted in 2 ml of WFI (USP Grade Invitrogen Cat A12873). 100 μl of the reconstituted peptide was removed and further diluted in 900 μl of WFI. 20 μL of the diluted peptide was retained for pot inoculation with the remaining volume aliquoted and stored at −80° C.
[0222]R...
example 2
Process for 226 ADV
[0254]Day −0
[0255]Buffer Preparation
[0256]IL-4
[0257]A 50 μg vial of IL-4 (USP grade, CellGenix cat 1003-050) was diluted with 250 μl of WFI (USP Grade Invitrogen Cat A12873) to produce a 200 μg / ml stock solution. The stock solution was stored at −80° C. with an aliquot being left for use in the pot inoculation
[0258]IL-7
[0259]A 50 μg vial of IL-4 (GMP, Cellgenix cat 1010-050) was diluted with 2004 of WFI (USP Grade Invitrogen Cat A12873) to produce a stock. The stock was diluted 1:25 with WFI to produce a working stock, which was then stored at −80° C. with an aliquot being left for use in the pot inoculation.
[0260]ADV PepTivator Peptide
[0261]A 60 nmol / peptide peptide (GMP PepTivator hexon V) was reconstituted in 2 ml of WFI (USP Grade Invitrogen Cat A12873). 100 μl of the reconstituted peptide was removed and further diluted in 900 μl of WFI. 20 μL of the diluted peptide was retained for pot inoculation with the remaining volume aliquoted and stored at −80° C.
[026...
example 3
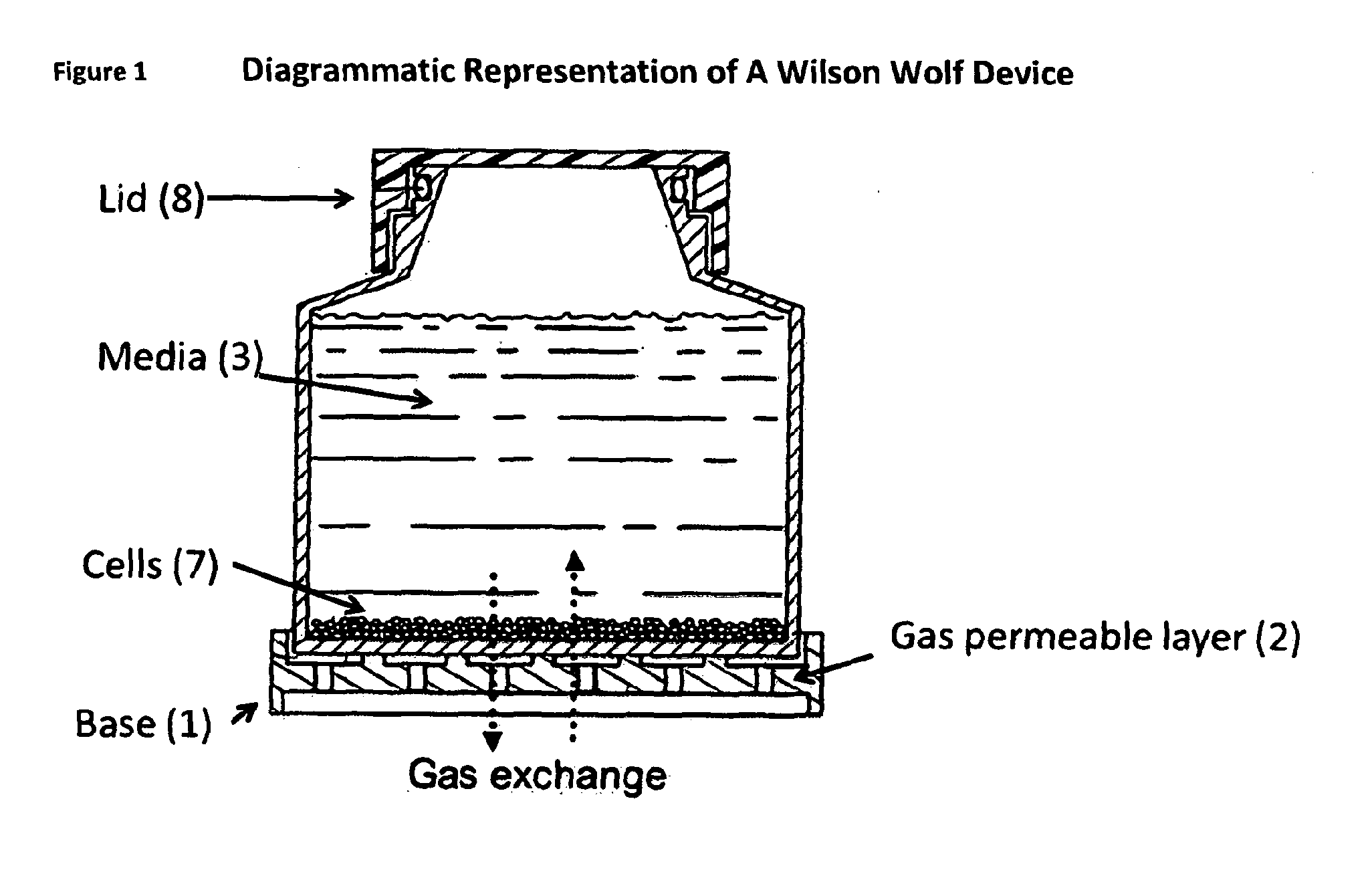
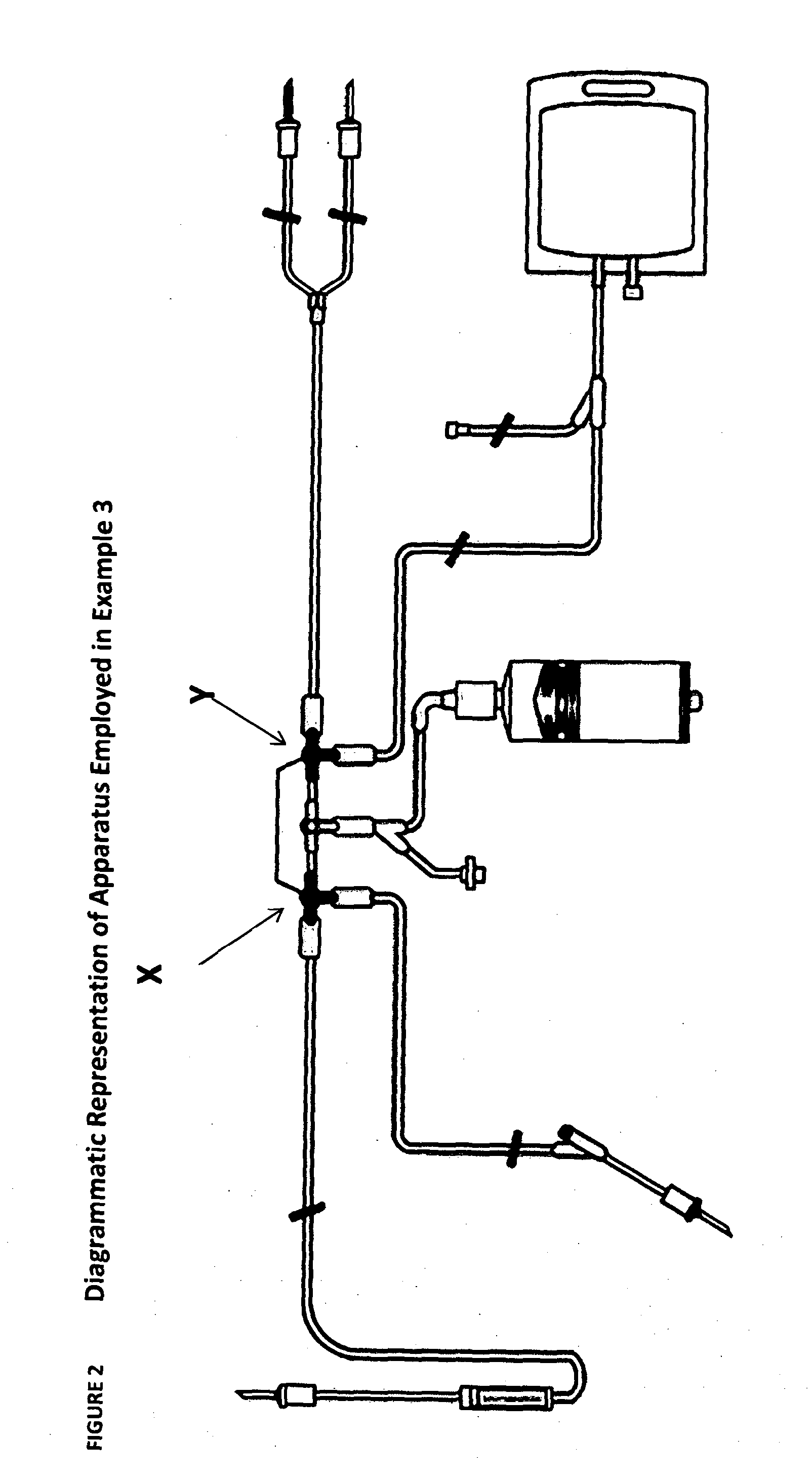
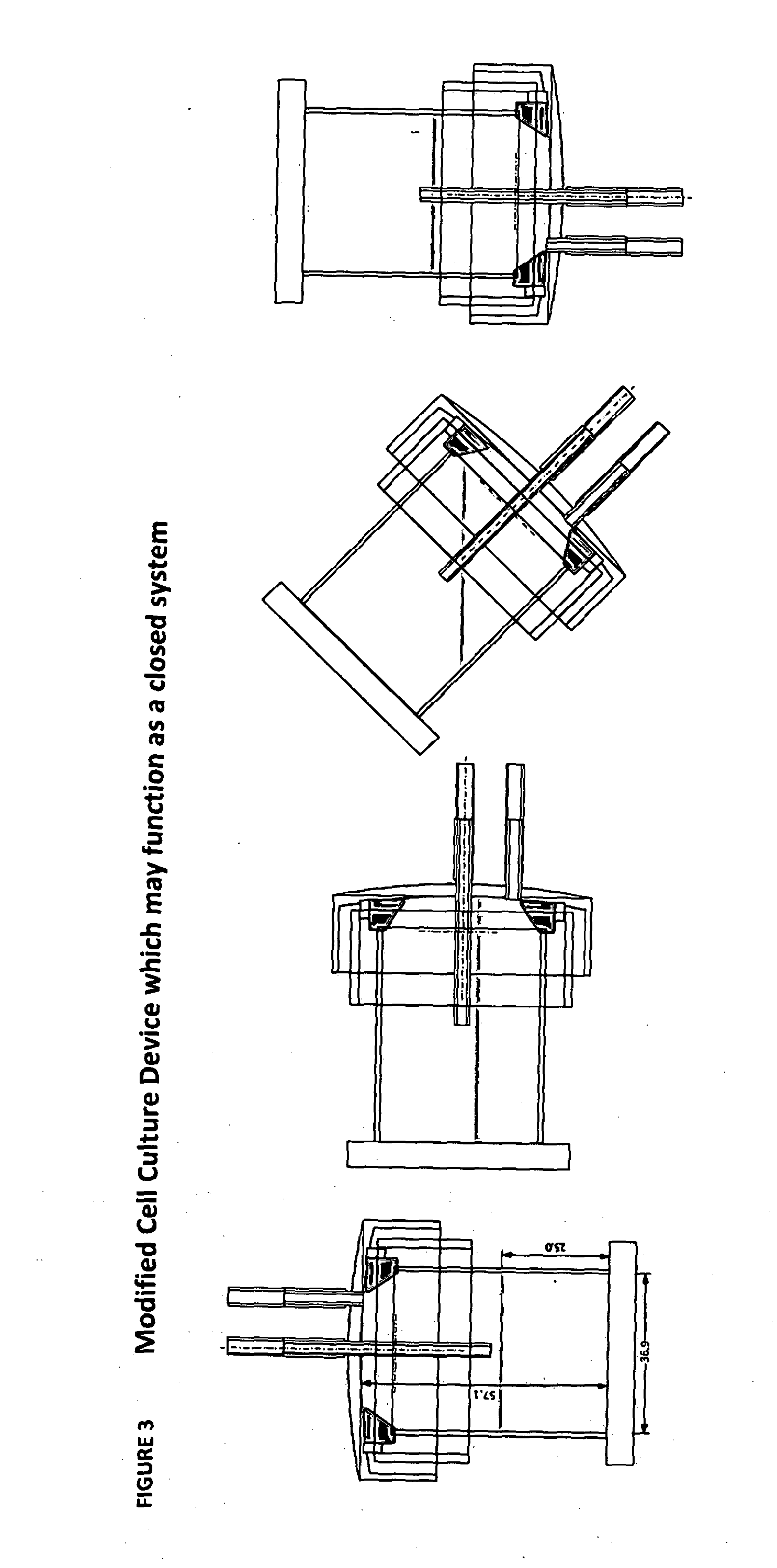
[0296]The starting population of cells was cultured in an adapted G-Rex system as shown in the Figures employing RPMI 1640 media in the presence of 10% human serum, IL4, IL7 and an overlapping peptide pool specific for the desired antigen. For CMV specific expansion then the peptide employed was pp65. For adenovirus (ADV) overlapping peptides for the ad 5 hexon were employed. A seed density of 0.5, 1 or 2×106 / cm2 was employed. The results established that 2×106 / cm2 lead to maximal cell expansion see Table 1 Seeding Density for CMV expansion:
Day 0Day 5Day 9Day 12Donor Ino cyto20000000183000001350000014100000IL4 / 72000000029100000108000000120000000no cyto10000000780000069000008100000IL4 / 710000000135000004350000081000000no cyto5000000180000021000001900000IL4 / 75000000420000064000003900000Donor IIno cyto20000000135000001500000022800000IL4 / 7200000001470000034500000120000000no cyto10000000450000055000005000000IL4 / 710000000125000003200000075000000no cyto5000000420000021000002700000IL4 / 750000...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com