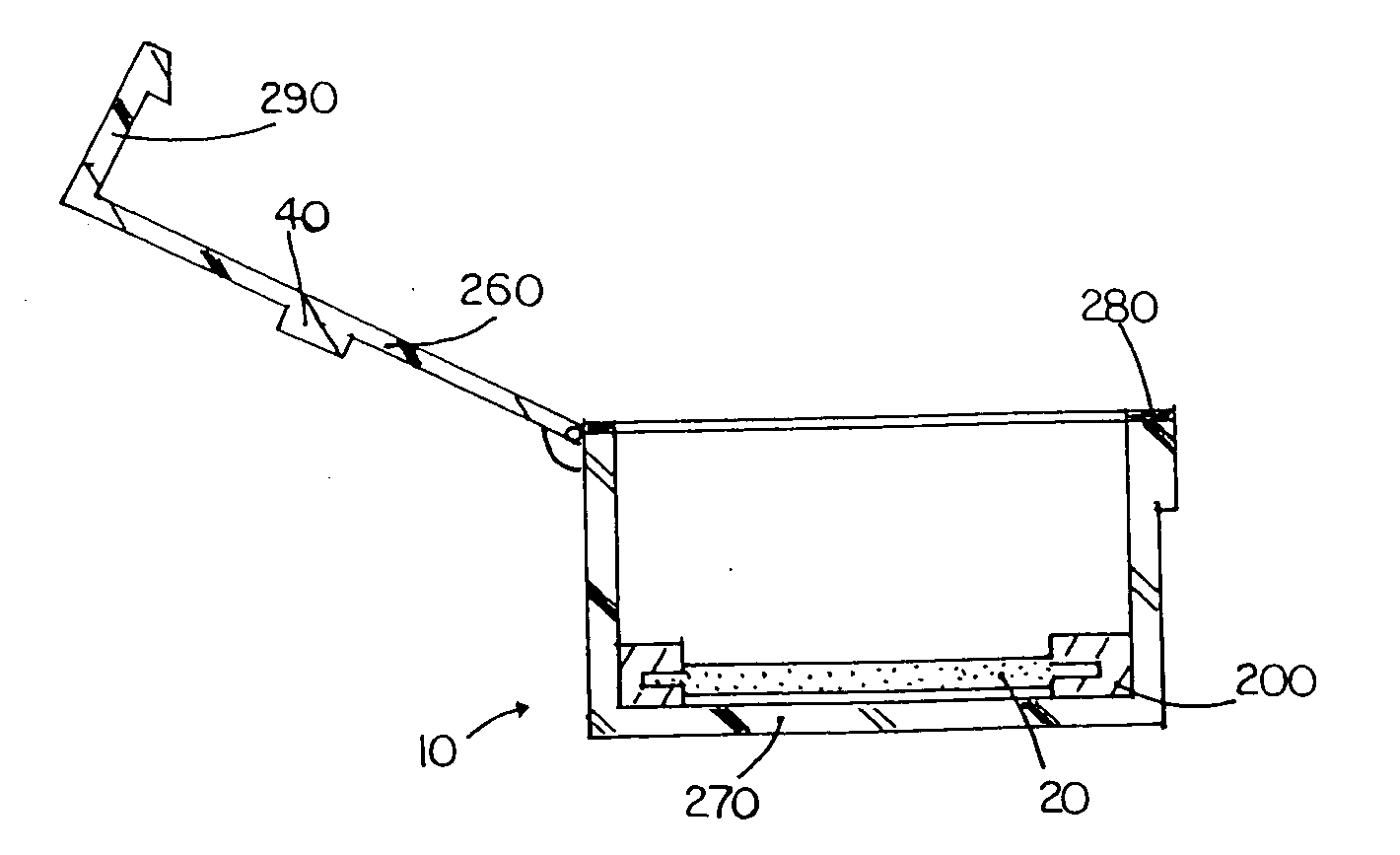
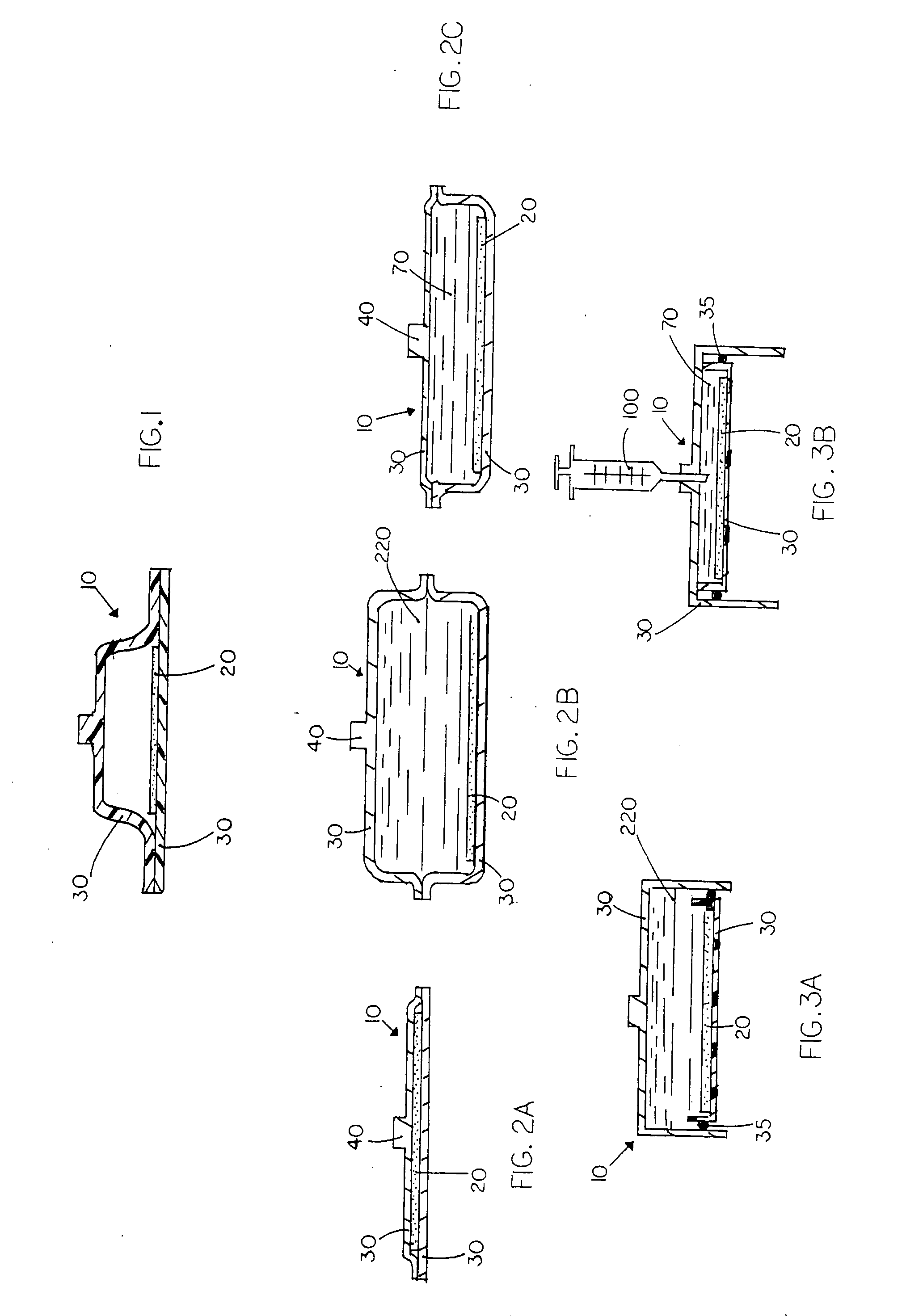
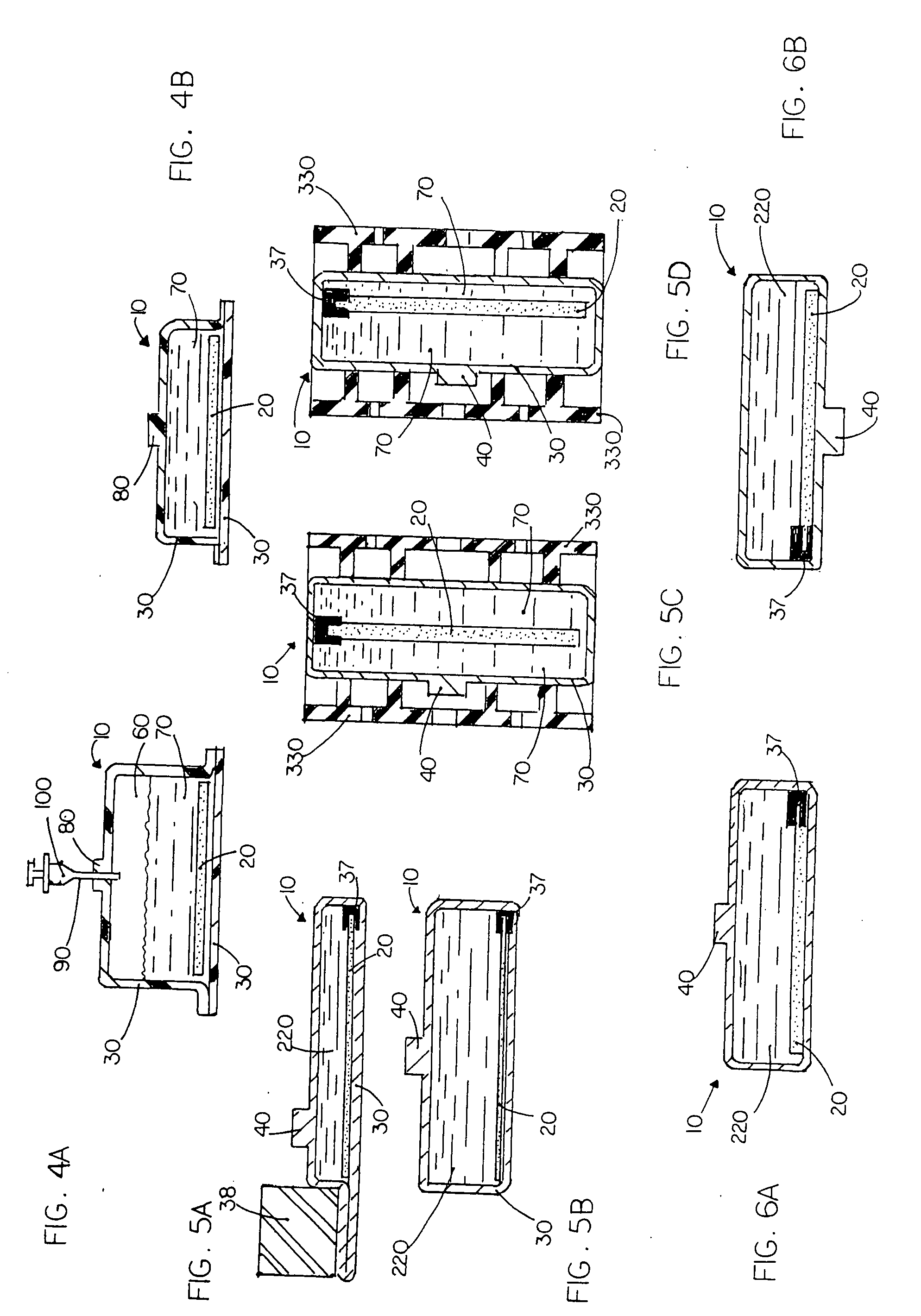
The
tissue culture device used for
tissue construct culture plays a critical role in the cost of production, which in turn impacts overall health care costs to society.
Unfortunately, the
petri dish does not provide optimal culture conditions or allow efficient production.
Another problem of the petri dish is related to
inoculation of
cell attachment matrices that facilitate three-dimensional culture such as collagen.
Cells residing on surfaces other than the cell attachment matrix can negatively
impact the culture.
Thus, the
inoculation procedure does not have tight
process control since cells must be directed, usually manually, to specific locations on the cell attachment matrix.
This problem is magnified as production is scaled up to produce more and more tissue constructs.
In this example, the petri dish is subject to
contamination due to the repeated handling needed for feeding.
The delivery of cells also creates contamination risk.
There is no way of controlling the amount of medium that resides on each side of the
sponge because that is dictated by the density of the
sponge relative to the density of the medium.
If humans are depositing the cells by way of pipetting or
syringe deliver,
skin constructs will exhibit a high degree of variance in the
initial distribution of cells, even when only one operator inoculates multiple sponges.
Robotic dispensation reduces the variance, but increases complexity and does not diminish the
exposure to contamination.
The petri dish is very poorly suited to protecting the
collagen sponge or helping it retain its desired shape.
This risks damage to the
sponge and fibroblasts and again exposes the sponge to contamination.
Thus, the shape of the sponge can change and there is no control over the final shape, a particularly undesirable characteristic when creating
skin constructs that may be laid side by side on a patient.
This leads to an additional handling process to
cut the sponge into a desired shape with all the risks of sponge damage and contamination present.
Long-term storage of the living
skin cannot be done in the petri dish because the materials are not compatible with freezing.
Subsequently, the bag must be sealed and filled with cryopreservatives, the process again risking damage to the cells and sponge, and risking contamination.
This also makes it difficult to perform
quality control in a manner that is inexpensive.
Even if non-destructive
process control limits were met during the culture process, such as glucose and
oxygen consumption targets, and those process control evaluations are not capable of detecting problems that occur once the culture is complete and the sponge is transferred to a cryopreservation bag.
If damage or contamination occurs at that point, it will be expensive to detect because the skin will have to be quarantined or a high amount of
destructive testing will be needed to verify the transfer procedures used for any given batch of skin were acceptable.
Another potential problem
in process control occurs because the amount of
cryoprotectant on each side of the sponge is a function of where the sponge comes to reside in the cryopreservation bag, over which the operator has little control.
Reconstituting the skin after cryopreservation can be done by removing the skin from the
cryoprotectant bag, placing it in a petri dish, and adding the appropriate medium to reconstitute it, thereby causing handling and contamination
exposure.
This example demonstrates that it is very difficult to establish tight process control for making
tissue construct products using the petri dish and a new apparatus and method is needed.
Attempts to address the limitations of the petri dish have been undertaken, but each attempt only addresses a portion of the problems and even when combined they do not lead to an alternative that has most of the desired attributes.
However, by applying them to the skin culture process described above, it can be seen that many problems remain.
This is too complex and costly for most research environments.
That increases
system complexity and cost and subjects cells to a higher rate of shear than may be desirable.
If
perfusion to bring
oxygen is not provided once the
oxygen in the
lower compartment is depleted, the lower portion of the tissue can only obtain oxygen from the medium in the upper compartment.
Thus, without high
flow perfusion, the device is no better than the petri dish for oxygenating cells at the bottom of the tissue.
In applications where the tissue is to be applied to a patient, such as living skin, Bell does not provide for a way of preparing the tissue without risking damage or increasing contamination risk.
Therefore, since techniques of
cutting the tissue are likely to vary from hospital to hospital, little process control is available.
However, that same characteristic can limit transport of desired molecules and compounds to the tissue from the surrounding medium.
Furthermore,
oxygenation of the culture is limited to
diffusion of oxygen from the upper liquid / gas interface.
The device does not lend itself to process control during scale up since it is not possible to measure the medium for indicators such as oxygen and glucose without taking individual samples from each device.
The
system is overly complex for tissue that is functionally adequate without being physically placed in tension.
Thus, at the research scale, the complexity and cost are prohibitive and unnecessary for many applications.
Even if the tissue loading elements are eliminated from the treatment chamber, the
system is still too complex for research applications as it relies on pumps and other
perfusion support mechanisms.
It also does not allow removal of the construct from its constraints without risking contamination of the treatment chamber and does not indicate how to prevent damage to the construct during the removal process.
However, whether or not the apparatus and method are applied to ligaments or some other tissue such as skin, many limitations of the petri dish remain.
Furthermore, the apparatus and method does not contemplate the need to protect the
bioreactor housing from damage during cryopreservation if the housing is comprised of a gas permeable material, capable of providing passive or non-passive
gas transfer to and from the culture, but not entirely compatible with cryopreservation conditions.
The apparatus and methods are also complex and eliminate the most desirable attribute of the petri dish, which is its simplicity.
 Login to View More
Login to View More