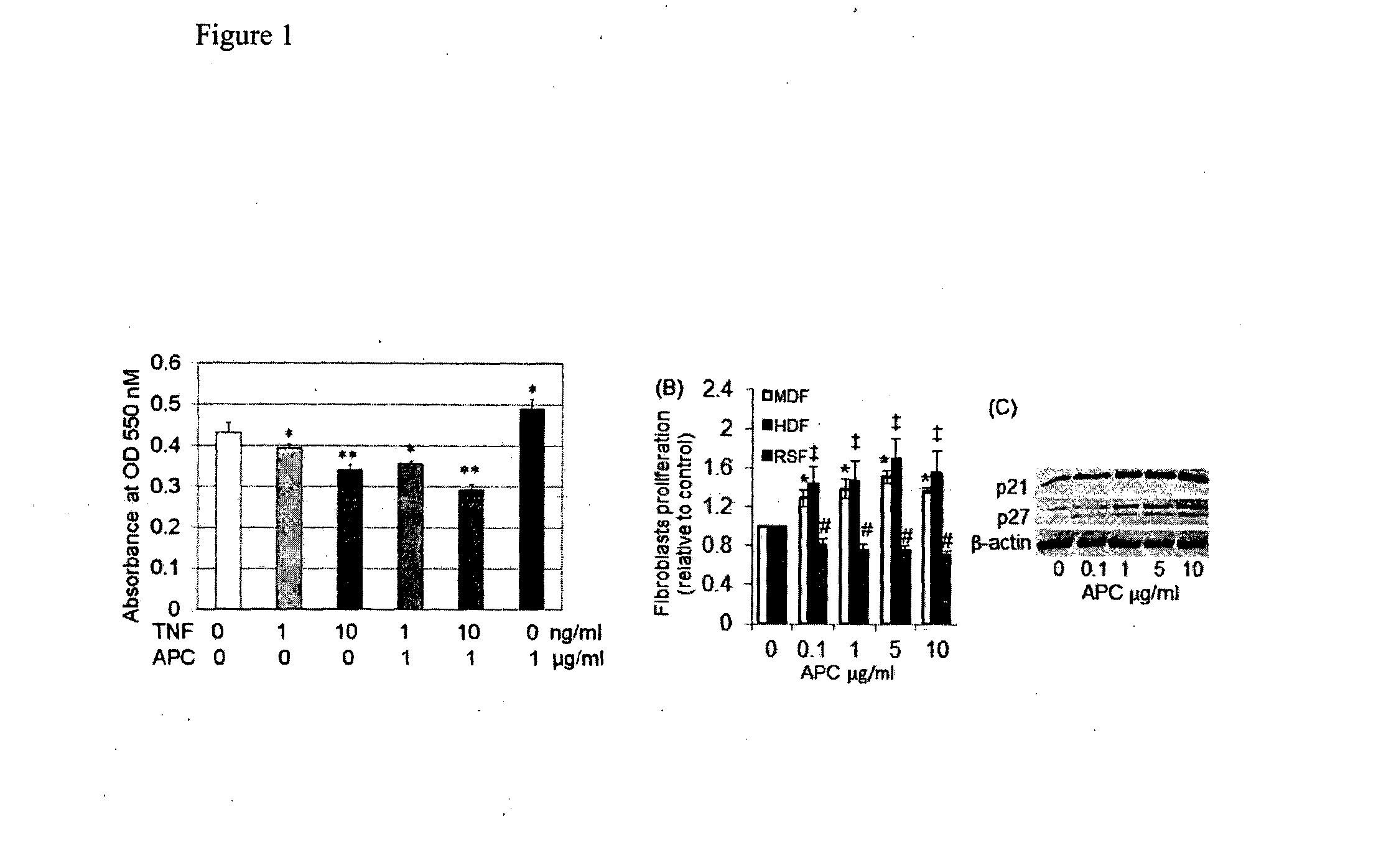
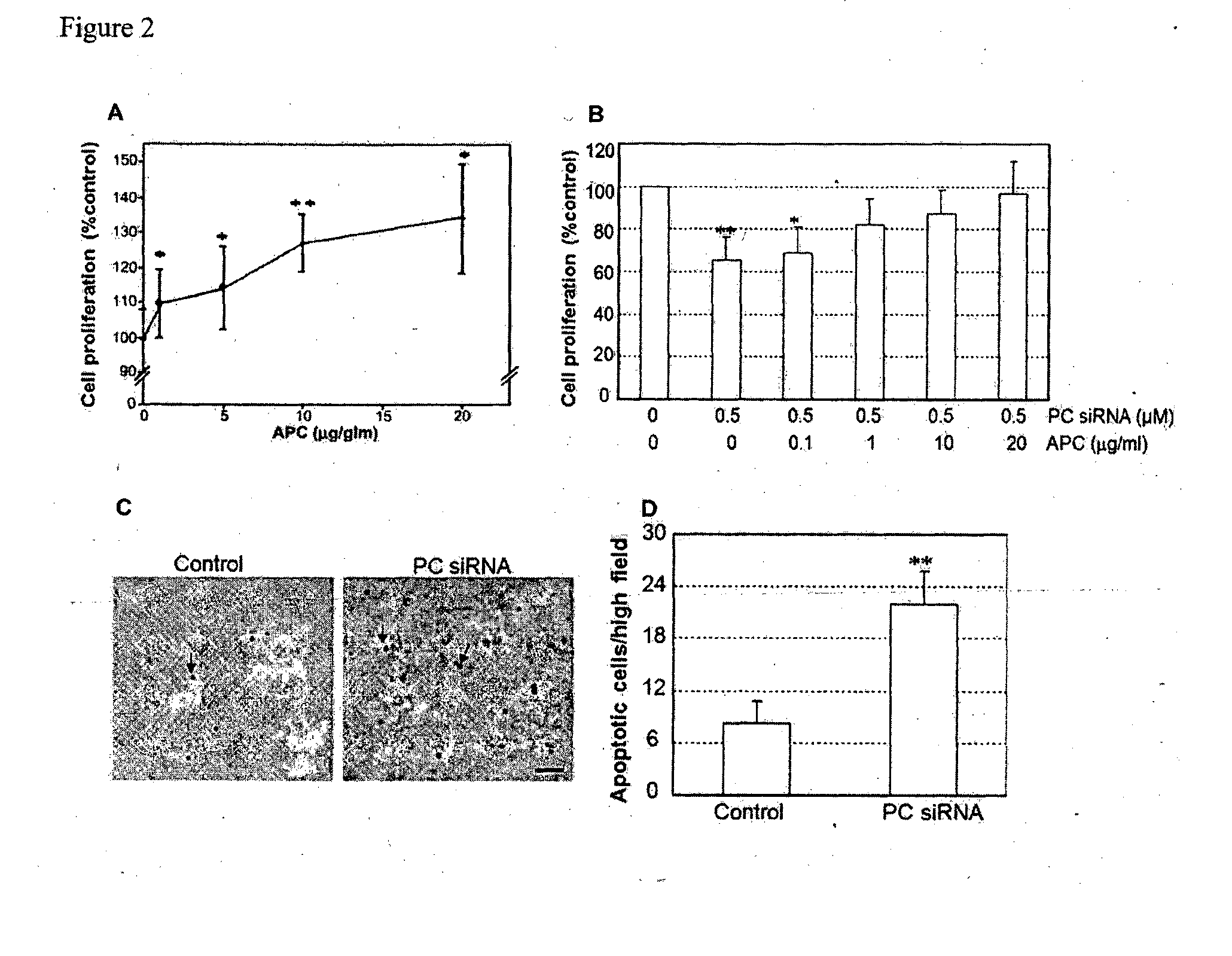
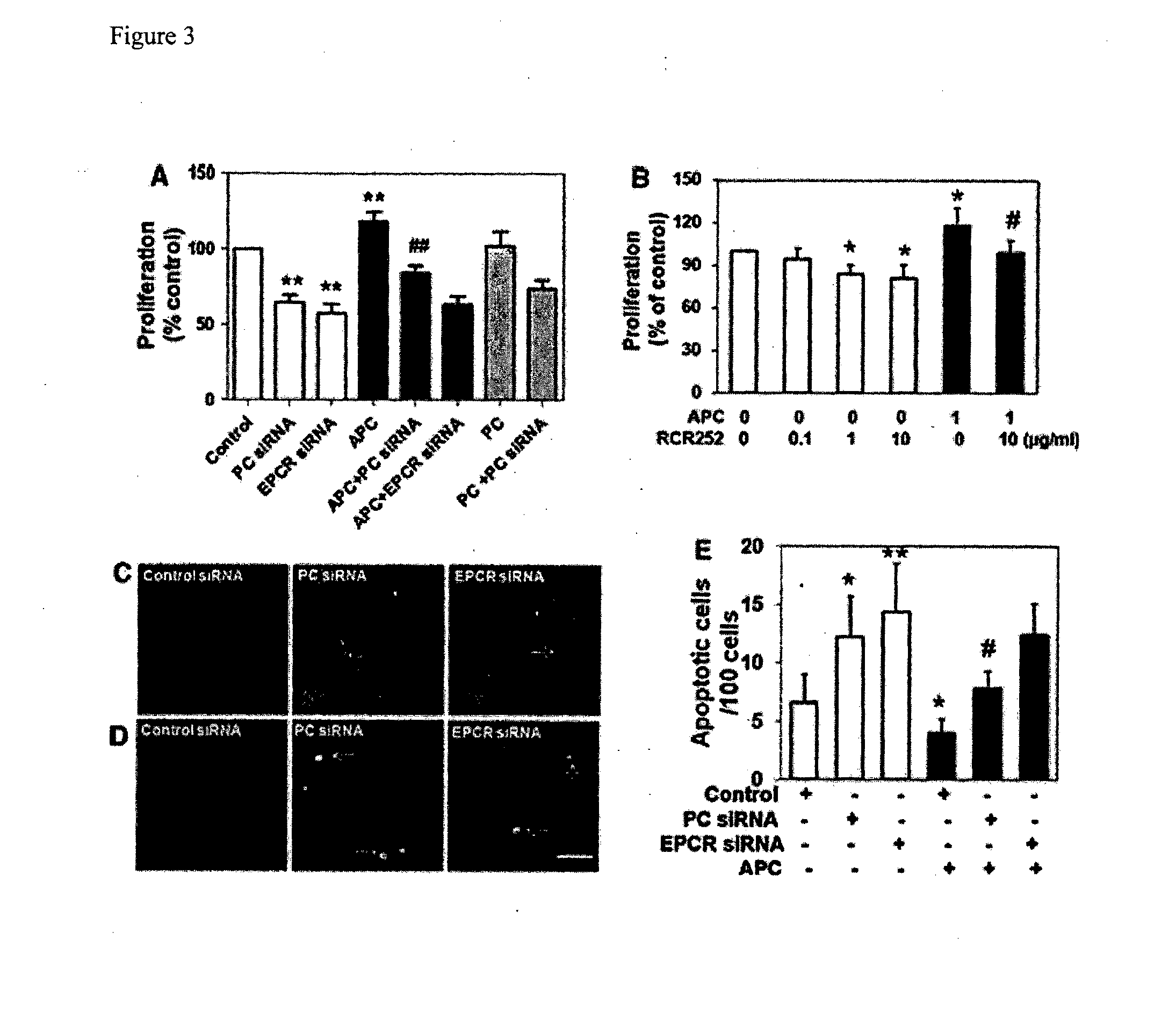
Treatment of inflammatory skin disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pre-Clinical Trial Establishing the Selective Anti-Apoptotic Activity of APC on Slow Growing, Non Inflammatory Keratinocytes and Psoriasis Skin Keratinocytes
[0135]Six patients with active chronic plaque psoriasis and 6 normal individuals are recruited. They have not received any treatment for at least 4 weeks prior to sampling. Two 6-mm punch biopsies are taken under local anesthetic from the same chronic plaque. Keratinocytes are isolated as we described previously (20).
[0136]Normal keratinocytes are treated with a mixture of cytokines [IL-1α (10 ng / ml), IL-6 (5 ng / ml), TNF-α (5 ng / ml), IL-17A (10 ng / ml)] to induce a psoriatic phenotype. APC is added to keratinocytes at 1, 10 μg / ml and treated for 24, 48 and 72 hrs. Cell proliferation / survival is examined using MIT assay, Brdu proliferation assay. Cell apoptosis is examined by TUNEL assay, Flow cytometry (propidium iodide (PI) or PI plus annexin-V). Cytokine production and four selected psoriasis-associated genes TNF, DEFB4, CAMP, ...
example 2
A Phase 2 Pilot Trial with Subcutaneous APC Over 12 Weeks
[0138]Patient selection: 5 Patients with severe chronic plaque-type psoriasis [Psoriasis Area and Severity Index (PASI)≧12, body surface area (BSA)≧10] are enrolled. Primary inclusion criteria includes patients 18 to 70 years of age who have had stable plaque psoriasis for at least 6 months. Primary exclusion criteria includes: i) patients with non-plaque or drug-induced psoriasis; ii) the use of biologicals such as rituximab, abatacept, infliximab, adalimumab, cyclosporine, or mycophenolic acid and etanercept or anakinra within 28 days prior to study iii) patients who have received anti-psoriatic treatment, including any phototherapy, during 8 weeks preceding the study and treatment with any standard topical therapy for psoriasis other than with bland emollients during 4 weeks preceding the study; iv) the use of topical therapy during the study is limited to class III to VII glucocorticoids on the scalp, axillae, and groin on...
example 3
A Phase 2 Pilot Trial with Subcutaneous aPC for Acne Vulgaris in 10 Patients
[0143]Patient selection: 10 Patients with acne vulgaris are enrolled. Primary inclusion criteria includes: patients 18 to 30 years of age who have had bilateral facial acne for at least 6 months. Primary exclusion criteria includes evidence of any active or recent infections, or a history of malignancy or other autoimmune disease, pregnant women.
[0144]Treatment: Patients are administered with 200 ug APC in gel form once a day for 12 weeks or when acne resolves. The patients are provided with two tubes of gel marked L and R and advised to apply 2 cm of gel, squeezed from the tube, to the designated affected area (L on left side of face and R on right side of face) and rub in evenly. Patients are then followed up for an additional 4 weeks after the final treatment.
[0145]Measurement: Photographs are taken before treatment and every 4 weeks and acne area calculated using computer-assisted image analysis. Adverse...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com