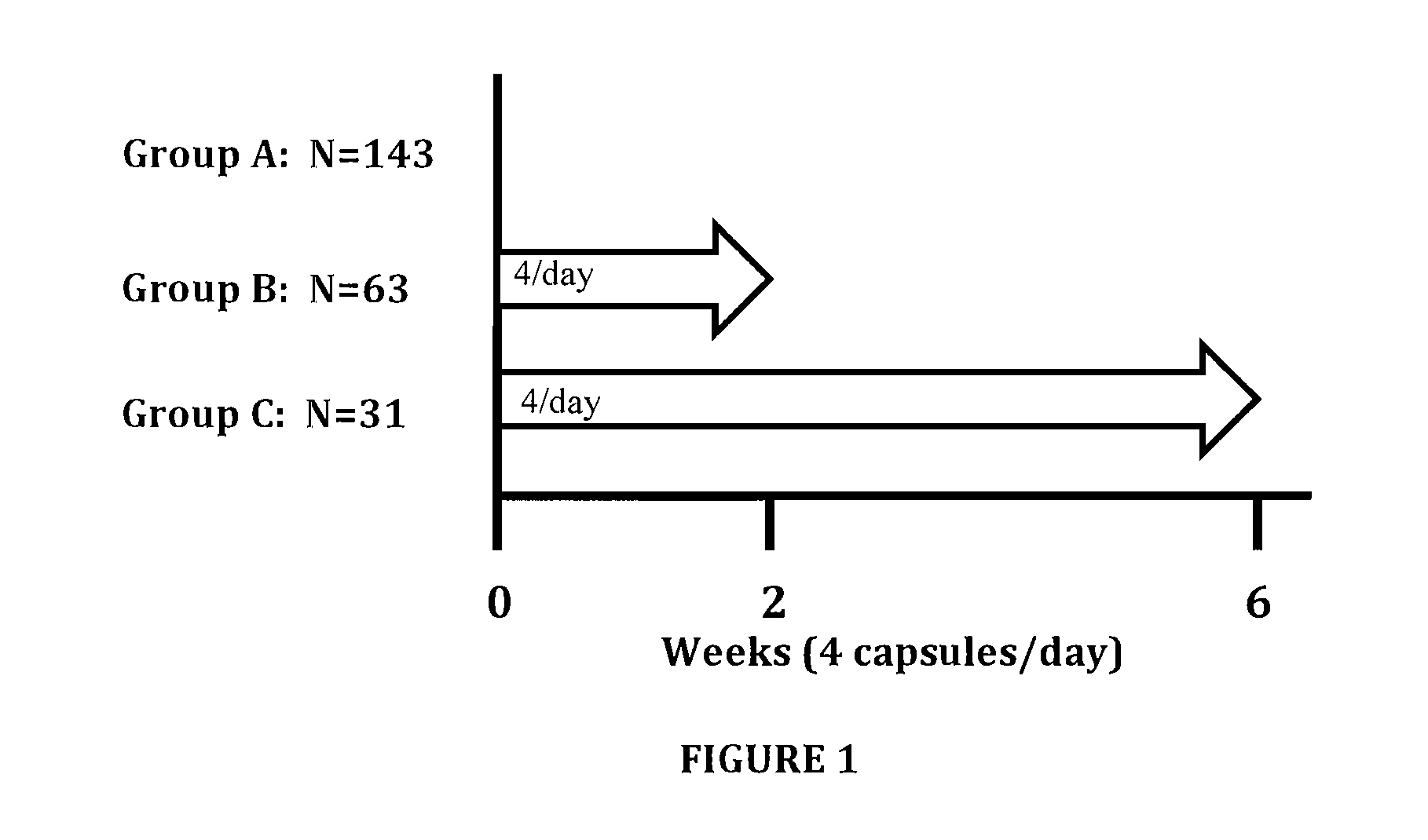
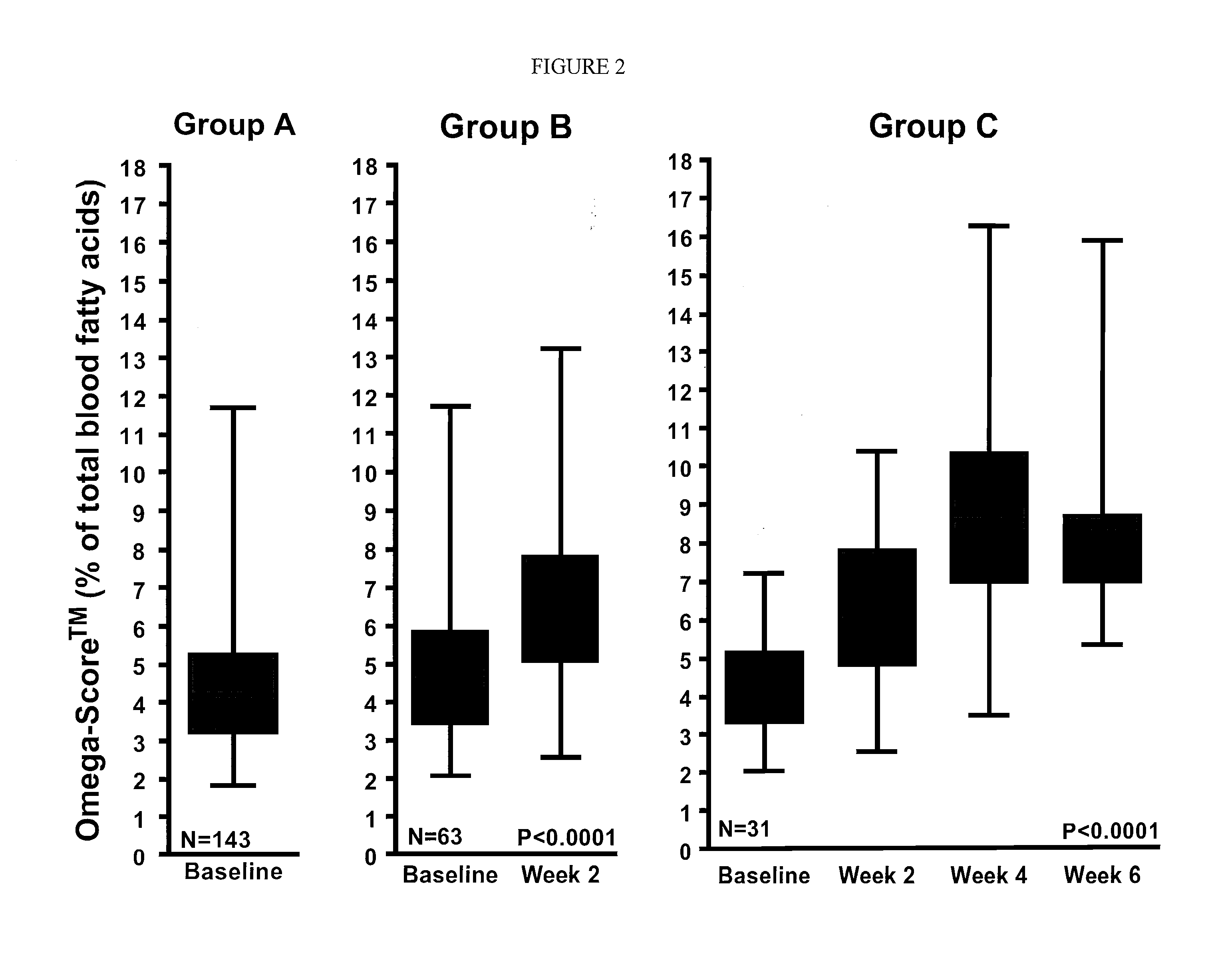
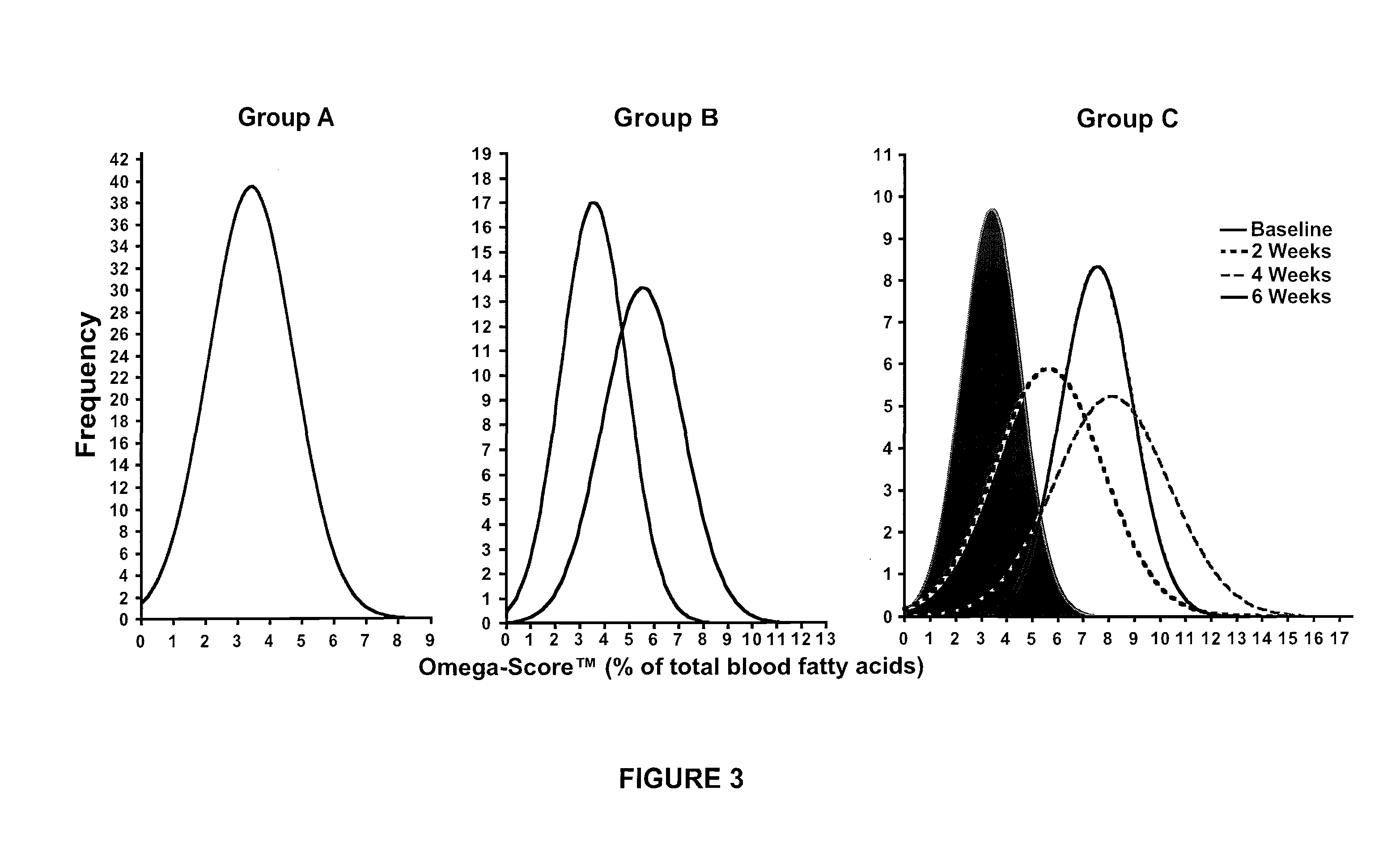
Omega 3 fatty acid for use as a prescription medical food and omega 3 fatty acid diagnostic assay for the dietary management of cardiovascular patients with cardiovascular disease (CVD) who are deficient in blood epa and DHA levels
a technology of omega 3 fatty acid and diagnostic assay, which is applied in the direction of biocide, plant growth regulator, pharmaceutical non-active ingredients, etc., can solve the problems of failing to teach or suggest the provision of companion diagnostic assays in the early art, and achieves enhanced vascular protective potential, reduced risk factors, and greater relaxation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044]The prescription medical food formulation provides a long chain fatty acid composition that includes a formulation containing a minimum of about 90% omega 3 fatty acids by weight having a combination of Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) in a weight ratio of EPA:DHA of from 5.7 to 6.3, wherein the sum of the EPA, DHA and DPA is about 82% by weight of the total formulation and about 92% of the total omega 3 fatty acid content of the composition. EPA+DHA are about 80% of the total formulation and about 89% of the total omega 3 fatty acid content of the composition. The fatty acids of the present invention are understood to include biologically active glyceride forms, e.g. triglycerides, biologically active ester forms, e.g. ethyl ester forms, and biologically active phospholipid forms, their derivatives, conjugates, precursors, and pharmaceutically acceptable salts and mixtures thereof.
[0045]The pharmaceutical formulation of the instant invention is conte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com