Ablation catheter system with safety features
a technology of safety features and catheters, applied in the field of catheters and systems, can solve the problems of ineffective pumping of blood into the ventricle, lack of synchrony, irregular and rapid heartbeat, etc., and achieve the effect of easy reconnection of connectors, easy separation of first and second connectors, and interrupting treatment or therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0216]A standard ablation catheter and a depressible red kill switch was spliced into the main conductor leading to the distal ablation electrode. The switch was positioned on the ablation handle for immediate thumb control, and was reassembled so that all the steerable components functioned according to design specifications. The system was then tested in vitro utilizing raw chicken and a standard approved RF ablation system.
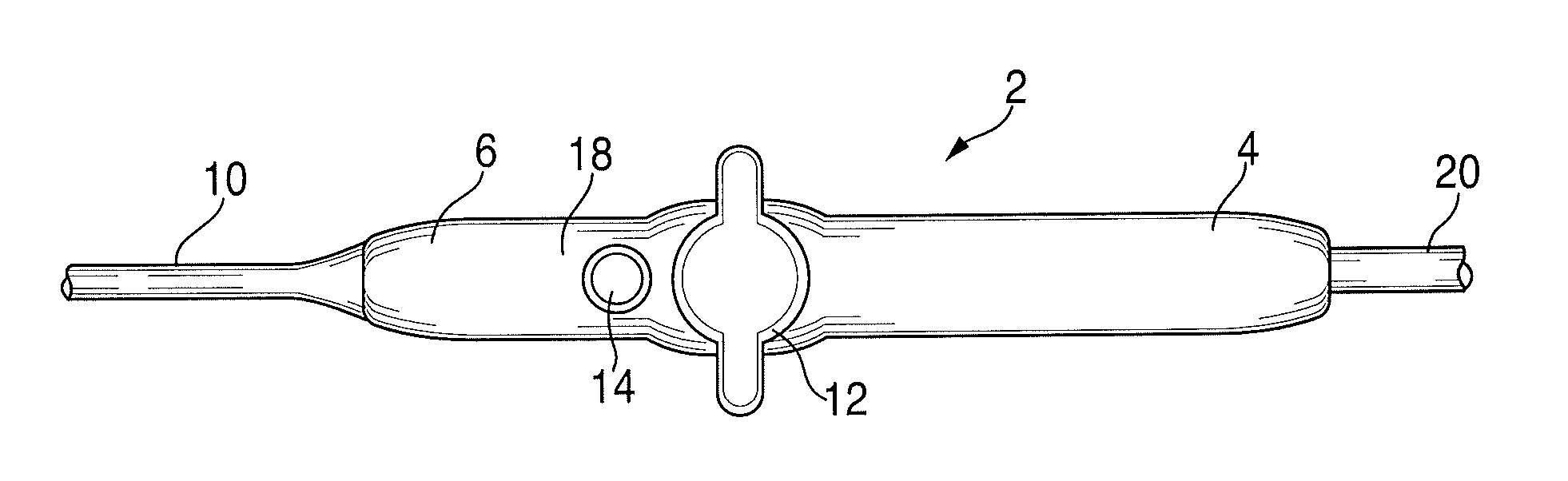
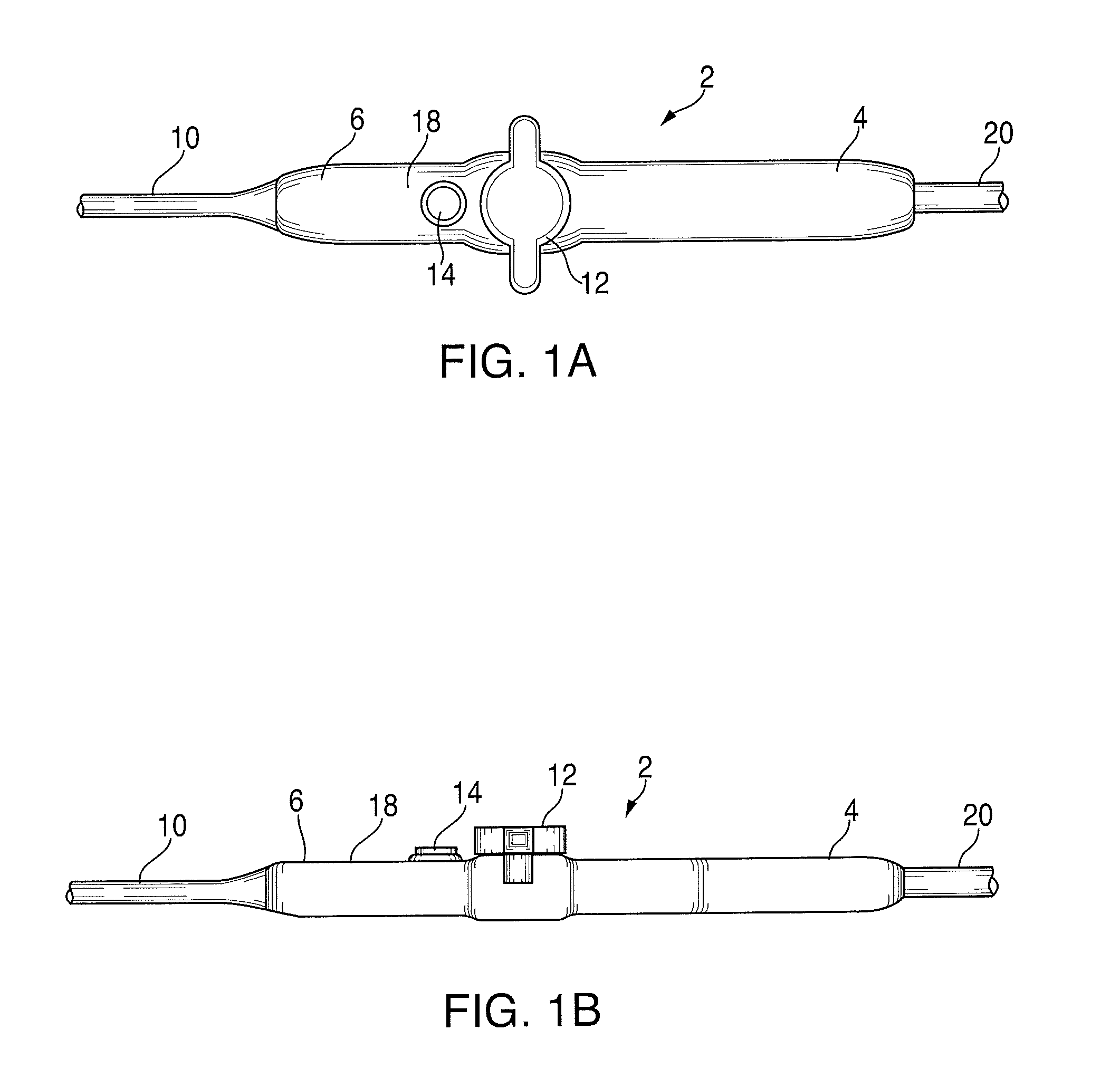
[0217]FIGS. 1A and 1B represent a radiofrequency ablation catheter system employing the kill switch on a standard handle, wherein, a standard ablation catheter was modified to include a red button as a kill switch. The system was tested multiple times and consistently created in vitro ablation lesions with precise manual control. Depressing the kill switch immediately terminated therapy delivery thereby preventing inadvertent radiofrequency delivery.
[0218]Conclusions: An ergonomic kill switch located in the thumb position on a standard ablation catheter handle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com