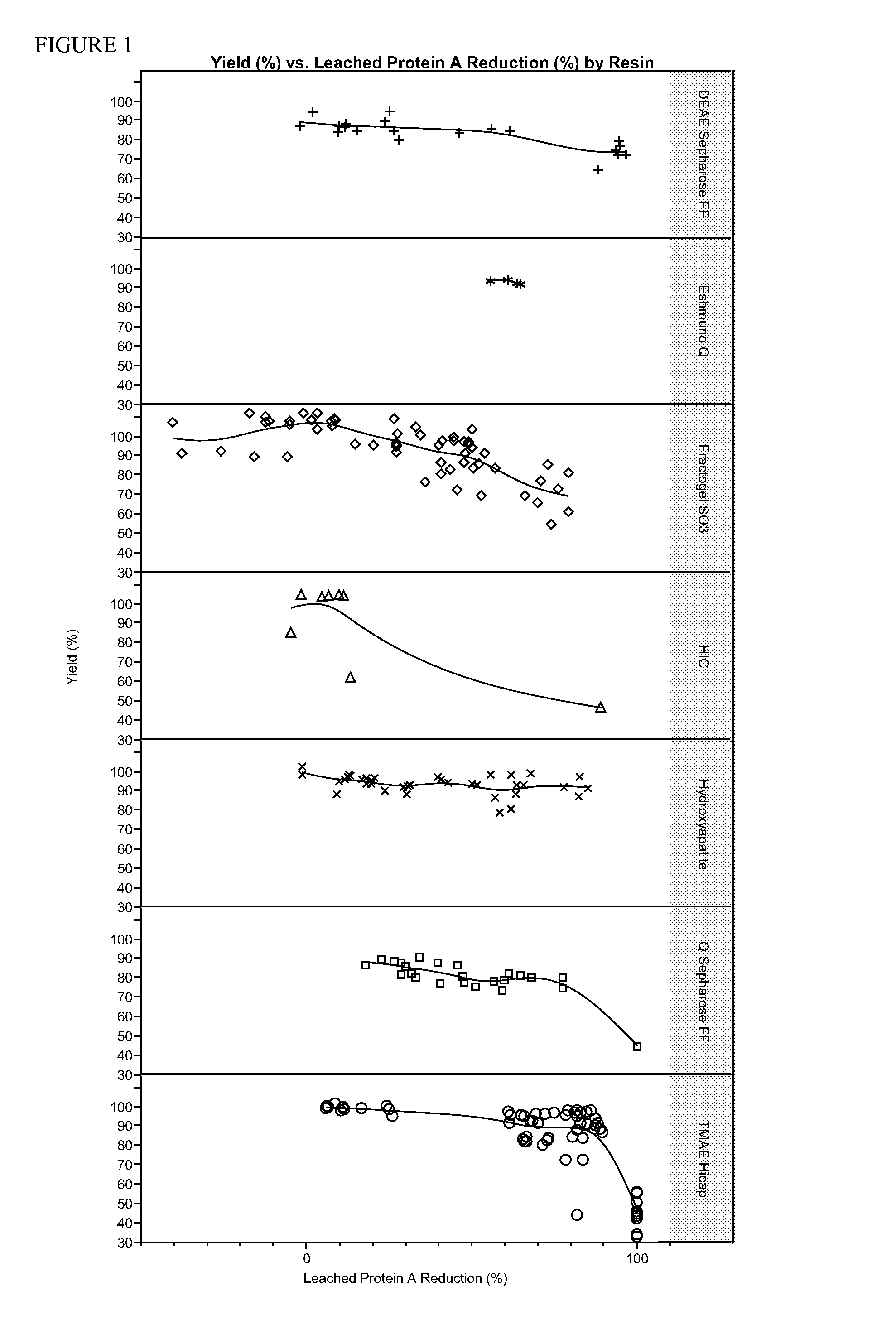
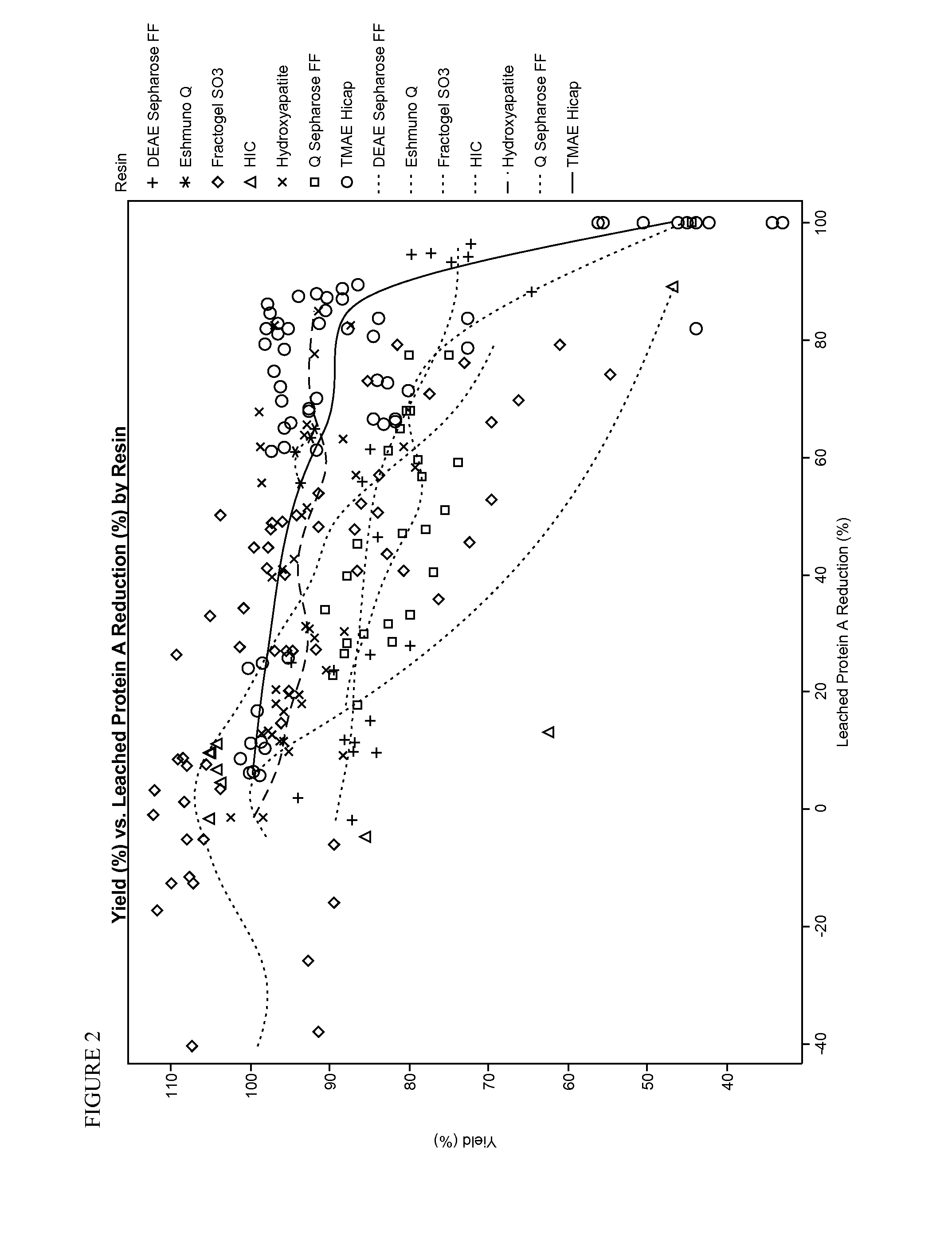
Removal of leaked affinity purification ligand
a technology of affinity chromatography and purification ligand, which is applied in the direction of peptides, drug compositions, separation processes, etc., can solve the problems of difficult to remove ligands from final preparations without, and ligand contamination, and achieves the effects of easy scaling, high efficiency, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0054]The goal of these experiments was to effectively remove contaminating Protein A from a recombinant protein preparation while recovering as much recombinant protein as possible. A recombinant protein, etanercept, was expressed in a transformed CHO (Chinese Hamster Ovary) cell culture, and secreted into the medium. After removal of cells from the medium, etanercept was initially purified by running the medium over a MabSelect SuRe™ Protein A resin column (GE Healthcare Life Sciences). Leached protein A was determined using a sandwich ELISA assay. Microtitre plates were coated with a chicken anti-protein A antibody as a capture antibody that was specifically raised against the MabSelect SuRe ligand. After blocking and washing steps, a biotinylated chicken anti-protein A antibody was used as the detection antibody. The amount of leached protein A in the sample after initial purification over the MabSelect SuRe™ Protein A resin column ranged from about 1 ppm to about 20 ppm.
[0055]I...
example 2
[0057]Using the relatively weak anion exchange resin DEAE Sepharose Fast Flow (GE Healthcare Life Sciences), a variety of different buffers and elution conditions was explored as detailed in the below table. Etanercept and protein A concentrations were determined as in Example 1.
Test Resin: DEAE Sepharose FFMode / ElutionLeachedEquilibrationLoadBufferProtein Aand WashCond.LoadElutionConductivityElutionYieldReductionBuffer(mS / cm)pHBuffer(mS / cm)Flow Rate(%)(%)Bind / Elute / 5.2825 mM Tris,17.140.5mL / min8027.925 mM Tris,150 mMpH 8NaCl, pH 7.4Bind / Elute / 5.2825 mM Tris,17.140.5mL / min8526.225 mM Tris,150 mMpH 8NaCl, pH 7.4Bind / Elute / 5.2825 mM Tris,200.5mL / min8515.125 mM Tris,175 mMpH 8NaCl, pH 7.5Bind / Elute / 5.2825 mM Tris,200.5mL / min8446.325 mM Tris,175 mMpH 8NaCl, pH 7.5Bind / Elute / 5.2825 mM Tris,13.70.5mL / min8655.925 mM Tris,125 mMpH 8NaCl, pH 7.5Bind / Elute / 5.2825 mM Tris,13.70.5mL / min8561.325 mM Tris,125 mMpH 8NaCl, pH 7.5Bind / Elute / 5.2825 mM Tris,13.30.5mL / min72.496.325 mM Tris,100 mMpH 8NaC...
example 3
[0059]In this experiment, a different anion exchange resin was tested for its ability to remove protein A while maintaining a high recovery rate. The resin was Eshmuno® Q, which is available from EMD Millipore, a division of Merck KGaA, Darmstadt, Germany. All runs were in a bind and elute mode.
Resin: Eshmuno QLeached ProteinEquilibrationLoad Cond.LoadElution BufferYieldA Reductionand Wash Buffer(mS / cm)pHand Conductivity(%)(%)25 mM Tris, pH 8,5825 mM Tris, pH 7.4,93.855.6cond. 5 mS / cmcond. 21 mS / cm25 mM Tris, pH 8,5825 mM Tris, pH 7.4,94.460.8cond. 5 mS / cmcond. 21 mS / cm25 mM Tris, pH 8,5825 mM Tris, pH 7.4,9264.7cond. 5 mS / cmcond. 21 mS / cm25 mM Tris, pH 8,5825 mM Tris, pH 7.4,92.563.3cond. 5 mS / cmcond. 21 mS / cm
[0060]Although the recovery of etanercept was high using this resin, reduction of Protein A was not sufficient.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com