Cardiovascular risk event prediction and uses thereof
a risk event and cardiac disease technology, applied in the field of cardiac disease risk event prediction, can solve the problem that the framingham equation is less useful for monitoring the change in risk of individuals, and achieve the effect of determining the risk associated with protein measurements more accurately and high cv events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Exemplary Biomarker Detection Using Aptamers
[0221]An exemplary method of detecting one or more biomarkers in a sample is described, e.g., in Kraemer et al., PLoS One 6(10): e26332, and is described below. Three different methods of quantification: microarray-based hybridization, a Luminex bead-based method, and qPCR, are described.
Reagents
[0222]HEPES, NaCl, KCl, EDTA, EGTA, MgCl2 and Tween-20 may be purchased, e.g., from Fisher Biosciences. Dextran sulfate sodium salt (DxSO4), nominally 8000 molecular weight, may be purchased, e.g., from AIC and is dialyzed against deionized water for at least 20 hours with one exchange. KOD EX DNA polymerase may be purchased, e.g., from VWR. Tetramethylammonium chloride and CAPSO may be purchased, e.g., from Sigma-Aldrich and streptavidin-phycoerythrin (SAPE) may be purchased, e.g., from Moss Inc. 4-(2-Aminoethyl)-benzenesulfonylfluoride hydrochloride (AEBSF) may be purchased, e.g., from Gold Biotechnology. Streptavidin-coated 96-well plates may be...
example 2
Methods
Study Design and Sample Collection
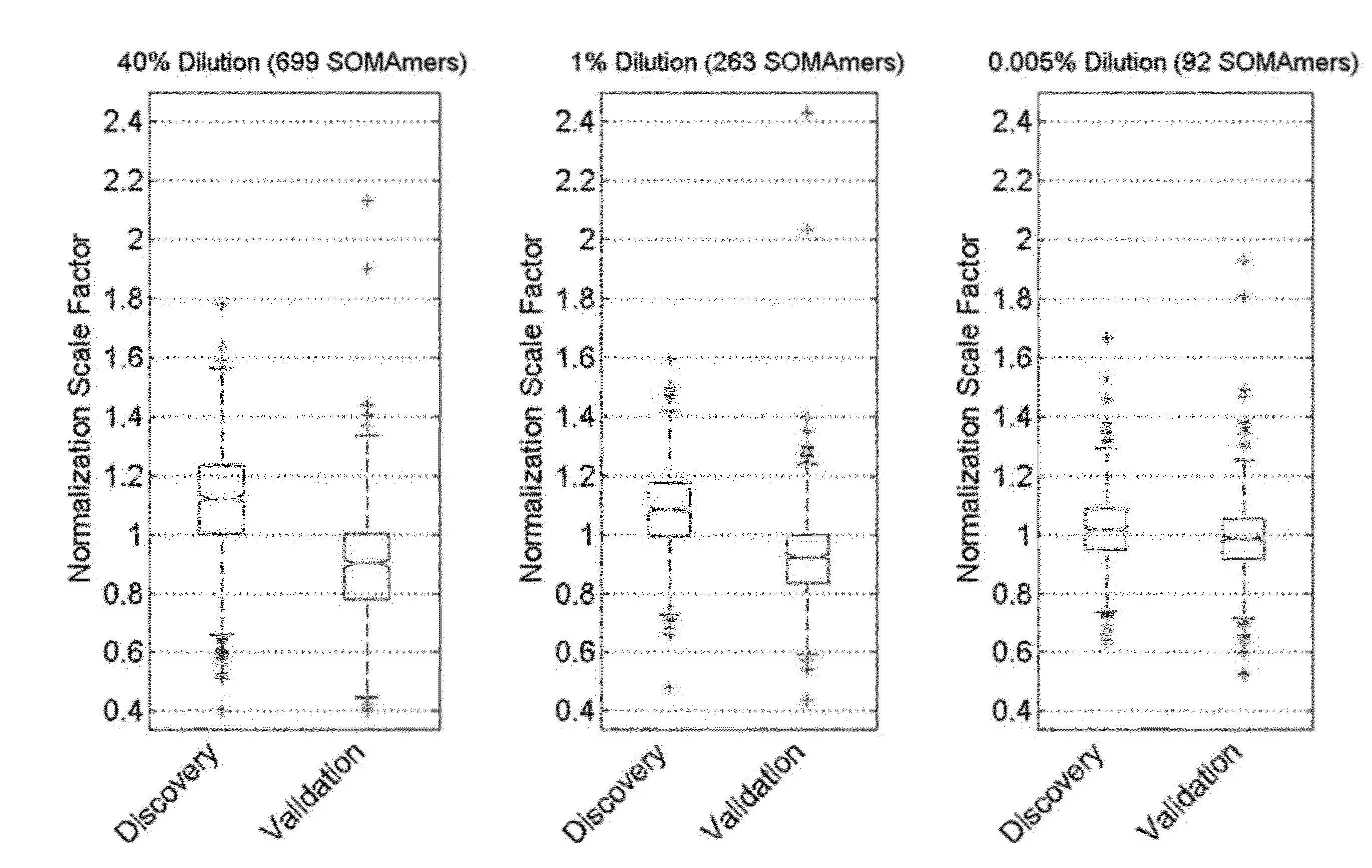
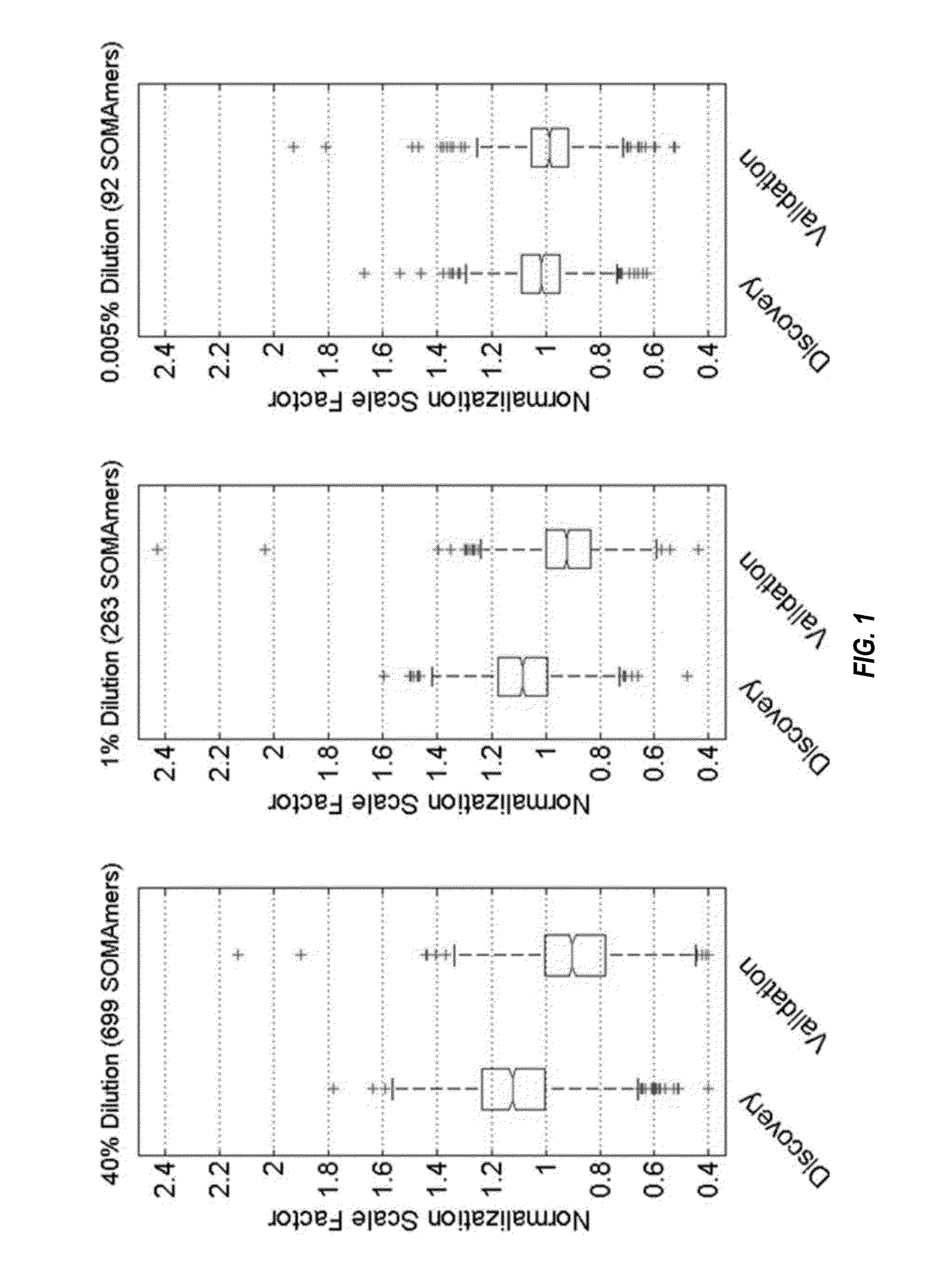
[0237]Archived plasma samples from subjects with stable CHD were obtained from two well-known, independent cohort studies. The characteristics of the study population are shown in Table 1. We performed protein biomarker discovery and model training in 938 plasma samples from the Heart and Soul study, with subsequent follow-up of 10 years. See, e.g., Shlipak et al., Am J Med. 2008; 121:50-57; Whooley et al., JAMA. 2008; 300:2379-2388. We validated the model on 971 samples from HUNT3, a Norwegian prospective cohort study with follow-up of 5 years. See Krokstad et al., Int Epidemiol. 2013; 42:968-977. We used the Heart and Soul inclusion and exclusion criteria to select all the participants with stable CHD from the larger HUNT3 cohort for this analysis. The discovery plasma samples were representative of a well-controlled academic prospective study: subjects were fasted, samples collected at the same time of day and centrifuged and frozen at −80...
example 3
Results
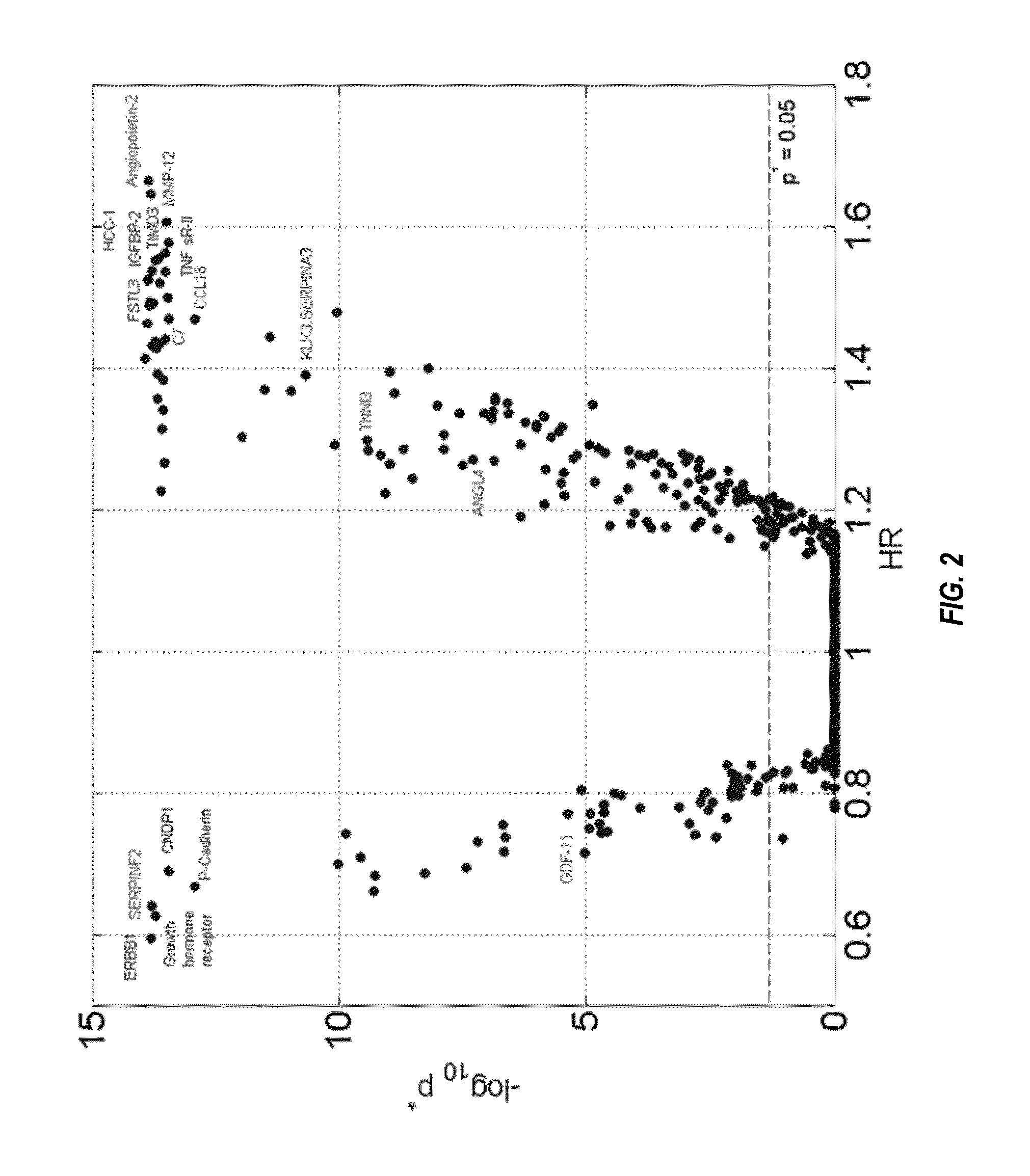
Baseline Characteristics
[0300]The clinical characteristics of the two study populations at baseline are summarized in Table 12. As expected, known risk factors are significantly more prevalent in the groups with events. There were fewer overall events in HUNT3 than in Heart and Soul, due to shorter follow up; nonetheless, the populations were generally comparable in the event rates per unit time and the distribution of the event types. In Table 12, P-values are associated with Fisher's exact test for categorical covariates and the Mann-Whitney U test for continuous covariates. Continuous values summarized with median and inter-quartile range (IQR). The HUNT3 validation set was not designed as a CHD study and as a result some clinical information was not available and is marked N / A. Legends: BMI=body mass index; ACE=angiotensin converting enzyme; ARB=angiotensin receptor blocker; LDL-C=low density lipoprotein cholesterol; HDL-C=high density lipoprotein cholesterol; TG=triglyce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com