Methods and devices for delivering drugs using drug-delivery or drug-coated guidewires
a technology of guidewires and drugs, applied in the field of medical devices, can solve the problems of body tissue showing adverse physiological reactions, tissue ischemia and necrosis, open heart surgery is, of course, very traumatic for patients,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
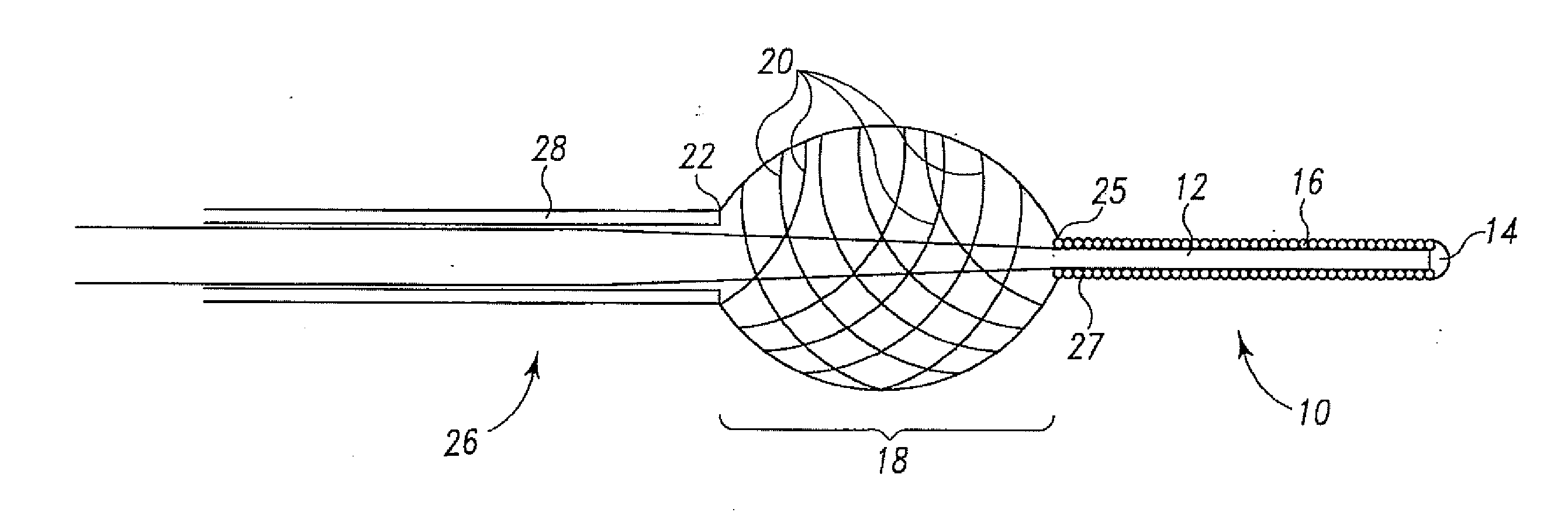
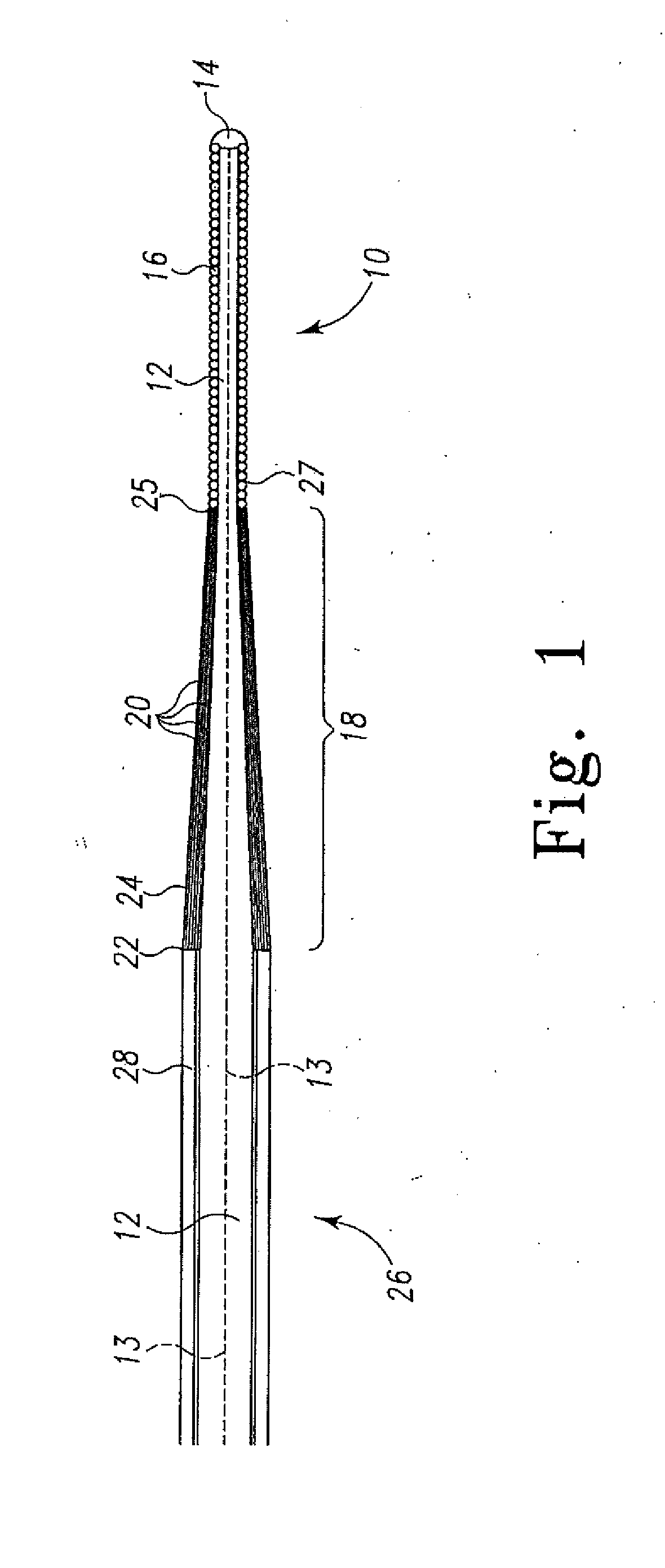
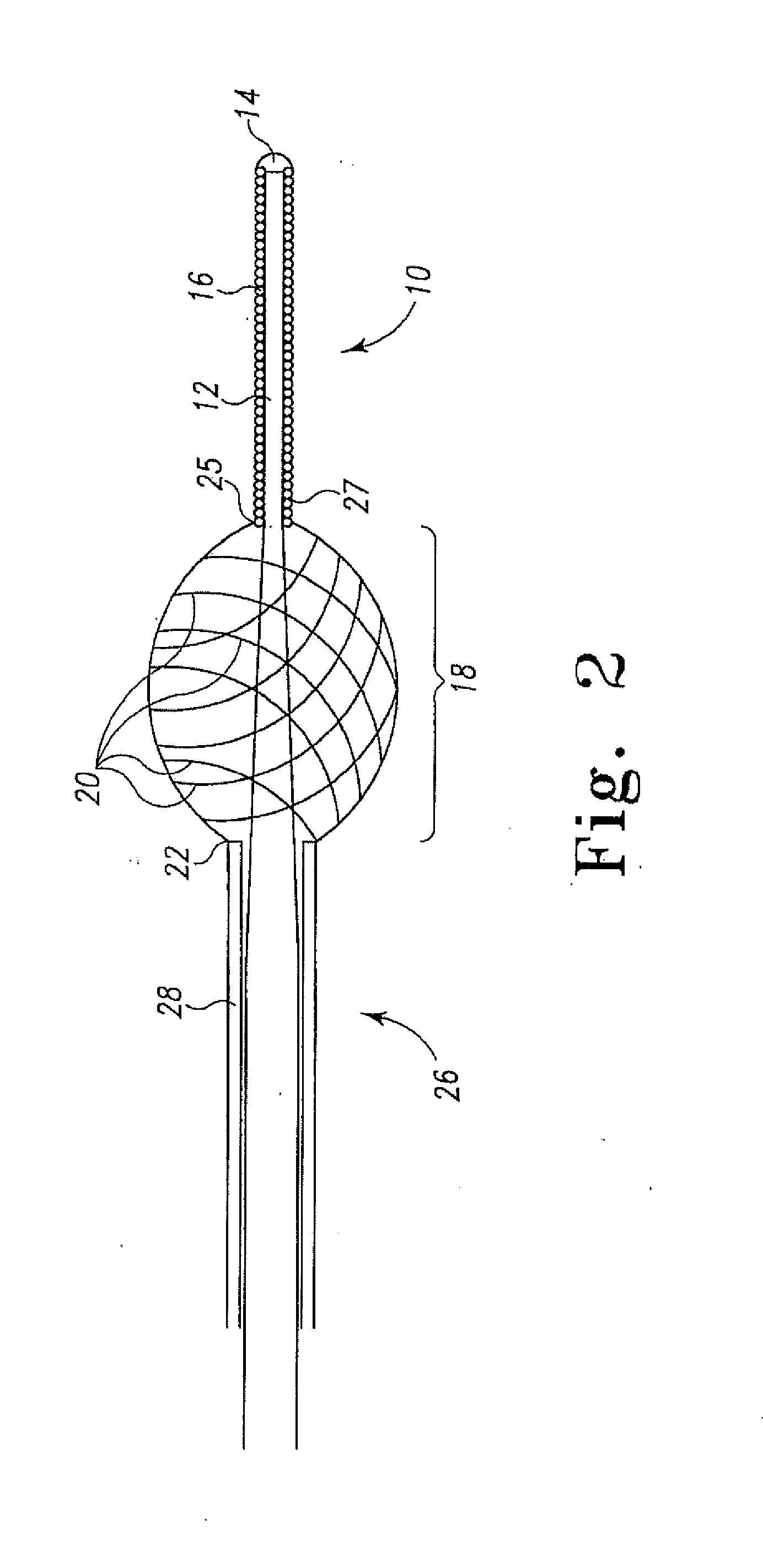
[0031]The present invention relates to a method of delivering drug(s), agent(s), cells or biological substances (the term “agent” includes drugs, biological substances, and biologics as those terms are used in their arts) in a target-specific manner, through the use of a drug or therapy-coated guidewire segment, portion or member, which includes drug delivery means and guidewire structure. The claimed method provides a therapy that targets the traumatized area by proximity alone or in combination with a systemic effect i.e. delivery from an exterior surface of a guidewire. A drug of the present invention provides, for example, anti-proliferative therapeutic activity to the cardiovascular system. A drug of this invention generally is effective locally, i.e., at the site of vessel contact, but may have more general systemic effects. A drug deployed by means of the present invention does not require a delayed or long term release and can be used, e.g., to activate anti-proliferative ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com