Methods and Devices for Delivering Drugs Using Drug-Delivery or Drug-Coated Guidewires
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
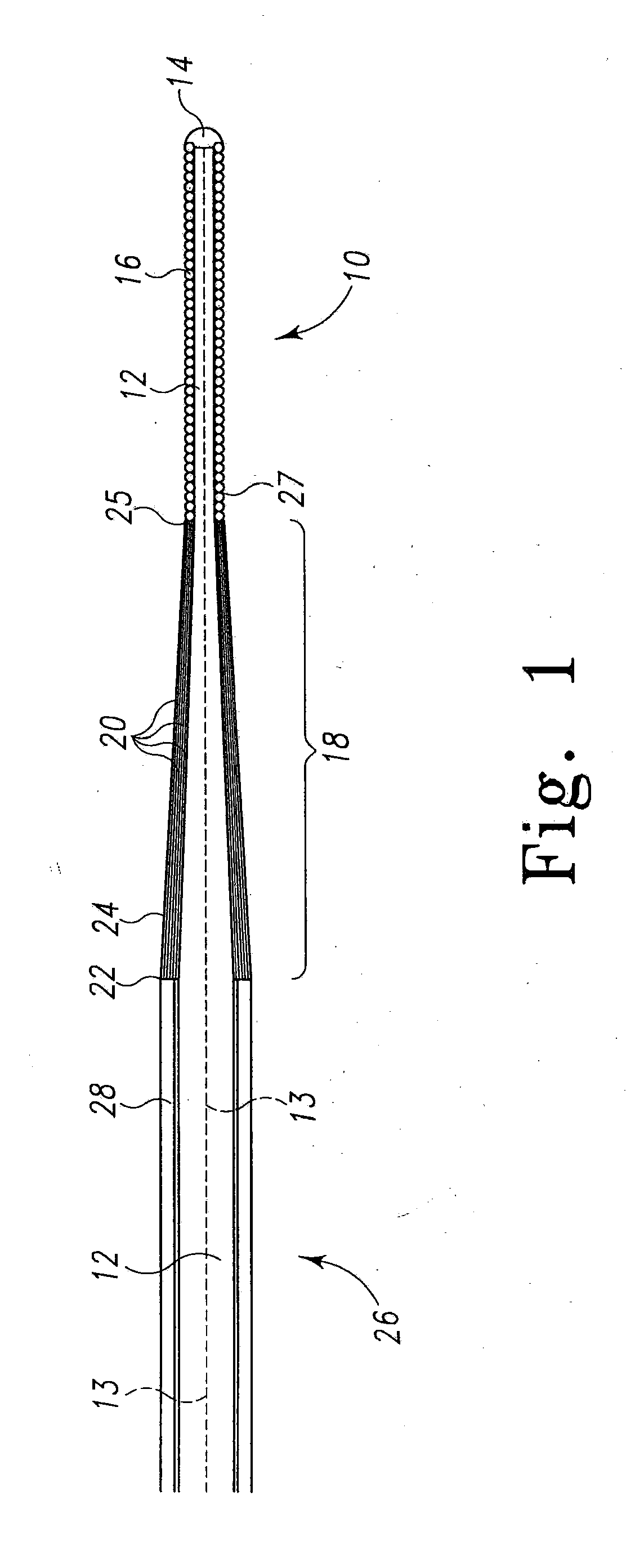
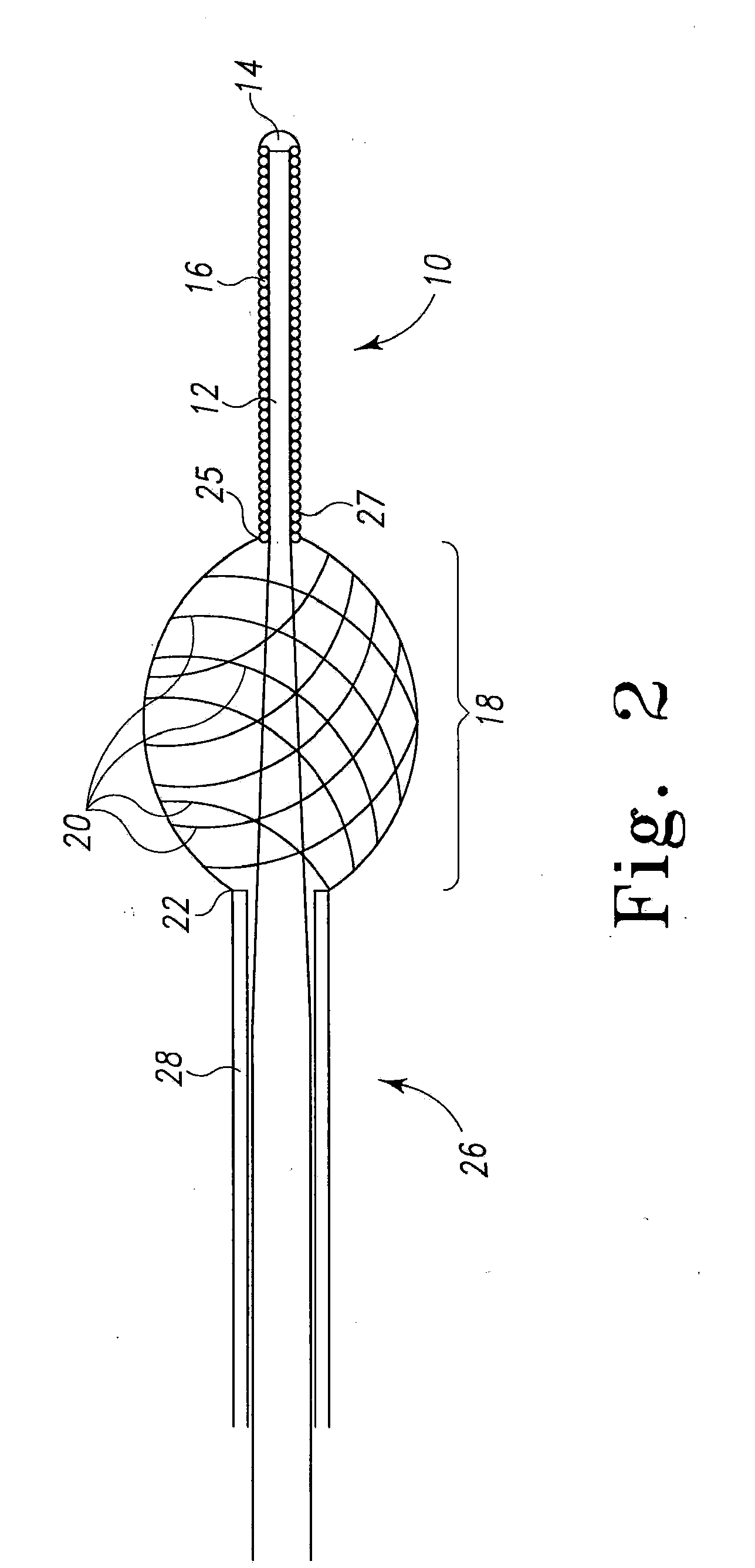
[0030]The present invention relates to a method of delivering drug(s), agent(s), cells or biological substances (the term “agent” includes drugs, biological substances, and biologics as those terms are used in their arts) in a target-specific manner, through the use of a drug or therapy-coated guidewire segment, portion or member, which includes drug delivery means and guidewire structure. The claimed method provides a therapy that targets the traumatized area by proximity alone or in combination with a systemic effect i.e. delivery from an exterior surface of a guidewire. A drug of the present invention provides, for example, anti-proliferative therapeutic activity to the cardiovascular system. A drug of this invention generally is effective locally, i.e., at the site of vessel contact, but may have more general systemic effects. A drug deployed by means of the present invention does not require a delayed or long term release and can be used, e.g., to activate anti-proliferative ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Biological properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com