Carboxamide derivatives and the use thereof as medicaments for the treatment of hepatitis b
a technology of carboxamide and derivatives, applied in the field of carboxamide derivatives, can solve the problems of lack of selectivity, poor efficacy, and limited direct treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
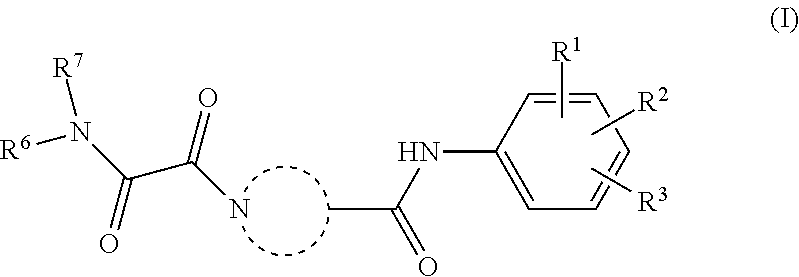
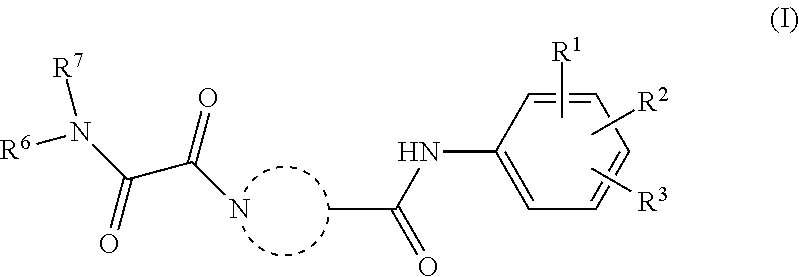
[0011]The present invention relates to a compound of Formula (I)
[0012]or a stereoisomer or tautomeric form thereof, wherein:
[0013]represents
[0014]each of Ra, Rb, Rc, Rd, Re, Rf and Rg are independently selected from the group consisting of Hydrogen and methyl;[0015]Rh is Hydrogen;[0016]Ri is Hydrogen;[0017]R1, R2and R3 are independently selected from the group consisting of Hydrogen, Fluoro, Chloro, Bromo, —CHF2, —CH2F, —CF3, —CN and methyl;[0018]R6 is selected from the group consisting of C1-C6alkyl and a 3-7 membered saturated ring optionally containing one or more heteroatoms each independently selected from the group consisting of O, S and N, such C1-C6alkyl or 3-7 membered saturated ring optionally substituted with one or more substituents selected from the group consisting of Fluoro, C1-C3alkyl optionally substituted with one or more Fluoro, —CN, OH;[0019]R7 represents hydrogen;
[0020]or a pharmaceutically acceptable salt or a solvate thereof.
[0021]The invention further relates...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com