High-purity carboxylic acid ester and method for producing same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
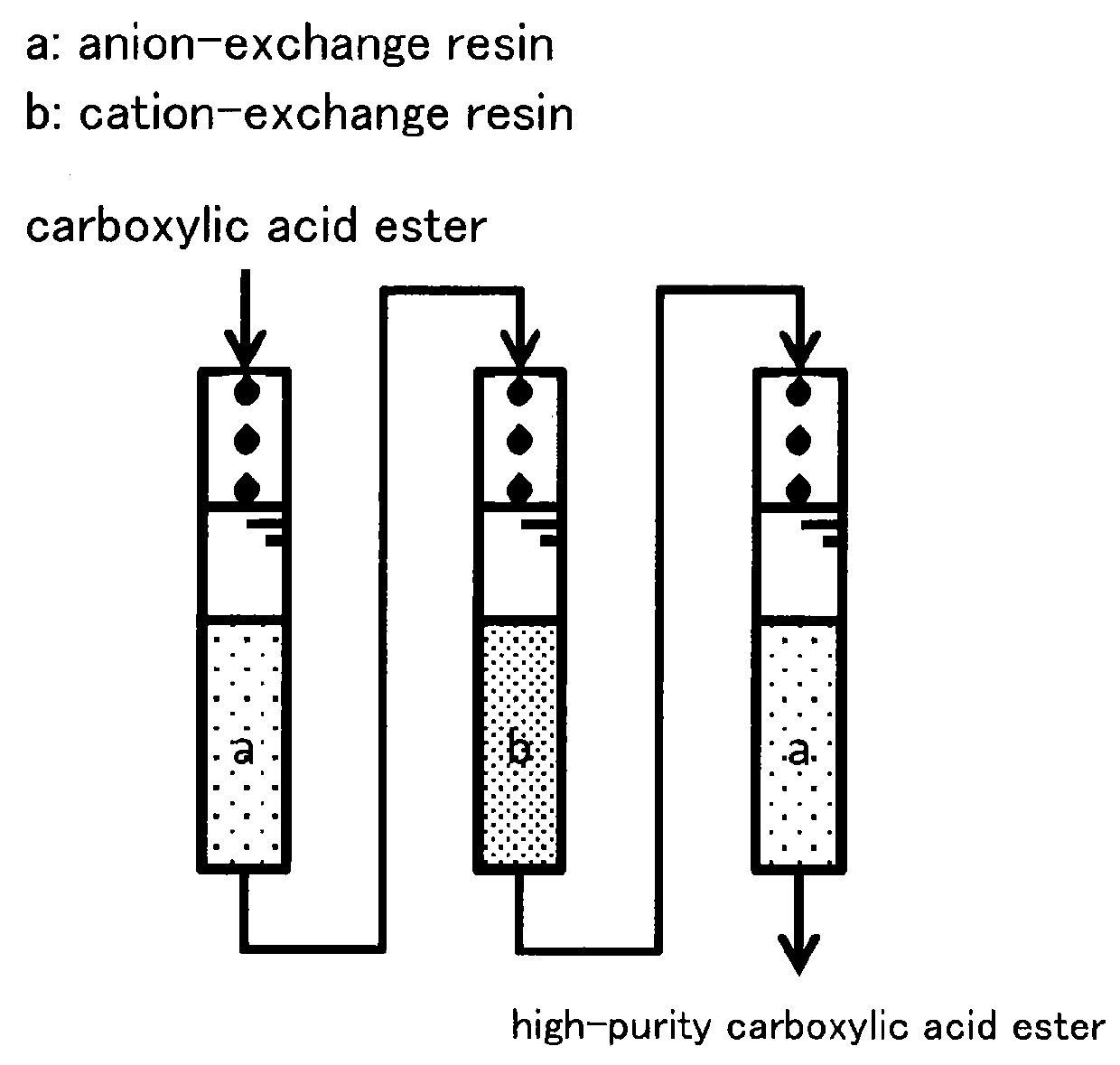
[0030]As a pretreatment, each of an H-type strongly acidic cation-exchange resin (trade name: 15JS-HG DRY, manufactured by Organo Corporation) and a free base type weakly basic anion-exchange resin (trade name: B20-HG DRY, manufactured by Organo Corporation) was put into ethyl lactate separately and immersed therein for 1 hour or longer while being gently stirred suitably. After that, one FEP column having an inner diameter of 16 mm was filled with 10 ml of strongly acidic cation-exchange resin, and each of two FEP columns having an inner diameter of 16 mm was filled with 10 ml of weakly basic anion-exchange resin. After that, ethyl lactate was flowed through the weakly basic anion-exchange resin (I), the strongly acidic cation-exchange resin (II) and the weakly basic anion-exchange resin (III) in this order at 25° C. with SV=20 Hr−1 as shown in FIG. 1. Respective concentrations of impurities after flowing through are shown in Table 1. From Table 1, it is understood that all the met...
example 2
[0031]As a pretreatment, each of an H-type strongly acidic cation-exchange resin (trade name: 15JS-HG DRY, manufactured by Organo Corporation) and a free base type weakly basic anion-exchange resin (trade name: B20-HG DRY, manufactured by Organo Corporation) was put into methyl hydroxyisobutyrate separately and immersed therein for 1 hour or longer while being gently stirred suitably. After that, one FEP column having an inner diameter of 16 mm was filled with 10 ml of strongly acidic cation-exchange resin, and each of two FEP columns having an inner diameter of 16 mm was filled with 10 ml of weakly basic anion-exchange resin. After that, methyl hydroxyisobutyrate was flowed through the weakly basic anion-exchange resin (I), the strongly acidic cation-exchange resin (II) and the weakly basic anion-exchange resin (III) in this order at 25° C. with SV=20 Hr−1 as shown in FIG. 1. Respective concentrations of impurities after flowing through are shown in Table 2. From Table 2, it is und...
example 3
[0033]An H-type strongly acidic cation-exchange resin (trade name: 15JS-HG DRY, manufactured by Organo Corporation) and a free base type weakly basic anion-exchange resin (trade name: B20-HG DRY, manufactured by Organo Corporation) were pretreated with methyl hydroxyisobutyrate in a manner similar to that in Example 2. After that, one FEP column having an inner diameter of 16 mm was filled with 10 ml of strongly acidic cation-exchange resin, and another FEP column having an inner diameter of 16 mm was filled with 10 ml of weakly basic anion-exchange resin. After that, methyl hydroxyisobutyrate was flowed through the strongly acidic cation-exchange resin (II) and the weakly basic anion-exchange resin (III) in this order at 25° C. with SV=20 Hr−1. Respective concentrations of impurities after flowing through are shown in Table 4. From Table 4, it is understood that all the metals described therein were highly removed. The anion content was highly removed during time between when flowi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com