Topical analgesic pain relief formulations, manufacture and methods of use thereof
a topical analgesic and composition technology, applied in the field of topical analgesic pain relief and antiinflammation compositions, can solve the problems of inability to use camphor as an analgesic compound, inability to achieve the effect of reducing nociceptive, neuropathic, and reducing nociceptiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
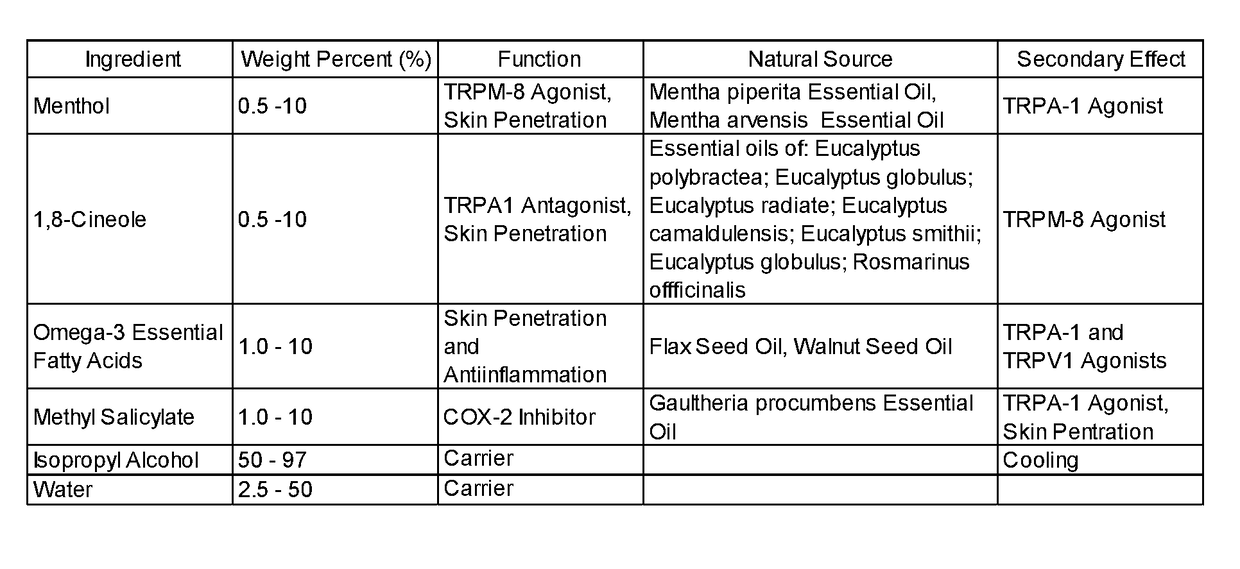
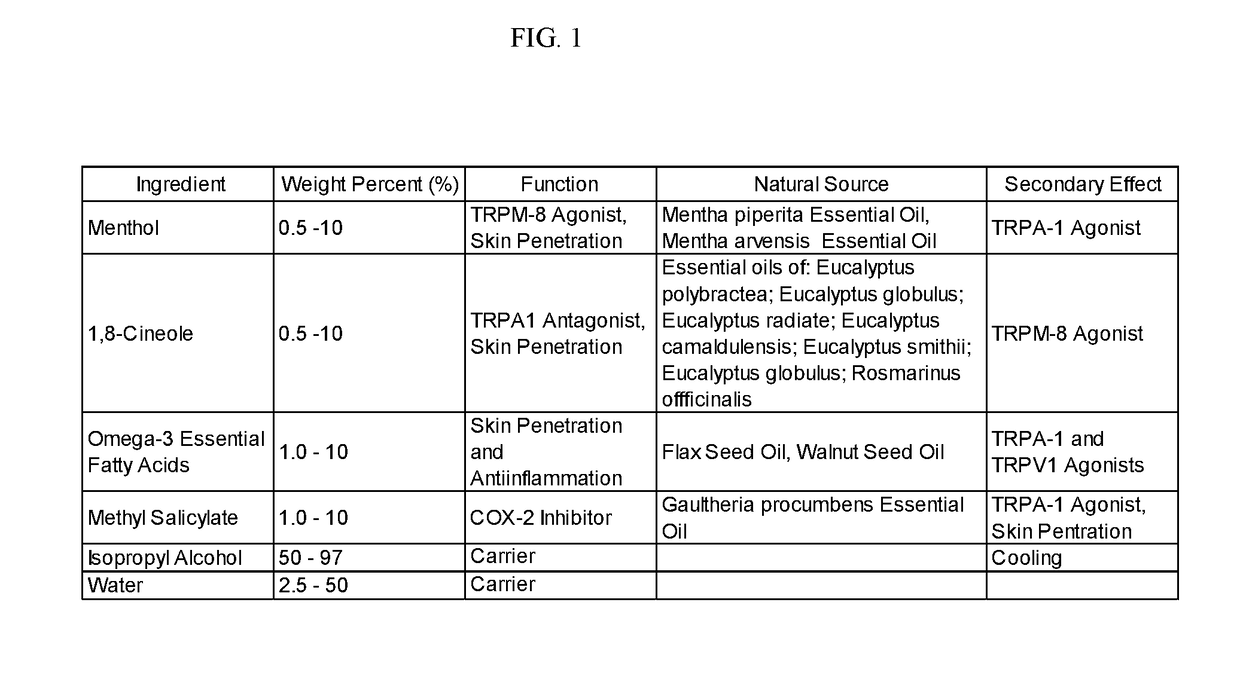
[0238]A method of manufacture of the topical analgesic sprayable liquid containing methyl salicylate, 1,8-cineole, menthol and Omega-3 fatty acids for the composition provided in Example 1 consists of mixing an amount of water with isopropyl alcohol with the other ingredients in a ratio of alcohol to water, such that a stable single phase homogeneous solution results. Mixing is limited to that required to create a stable single phase homogeneous solution and to minimize volatilization of menthol and 1,8-cineole. Methods of use of the composition of the topical analgesic given in Example 1 include but are not meant to be limited to placing the composition in a spray bottle and spraying onto the skin, placing the composition in a closed aerosol spray vessel under pressure of an inert gas and spraying on the skin and wetting a patch a placing on the skin. The topical analgesic sprayable liquid composition is Example 1 can optionally be made with borneol or a mixture of 1,8-cineole and ...
example 2
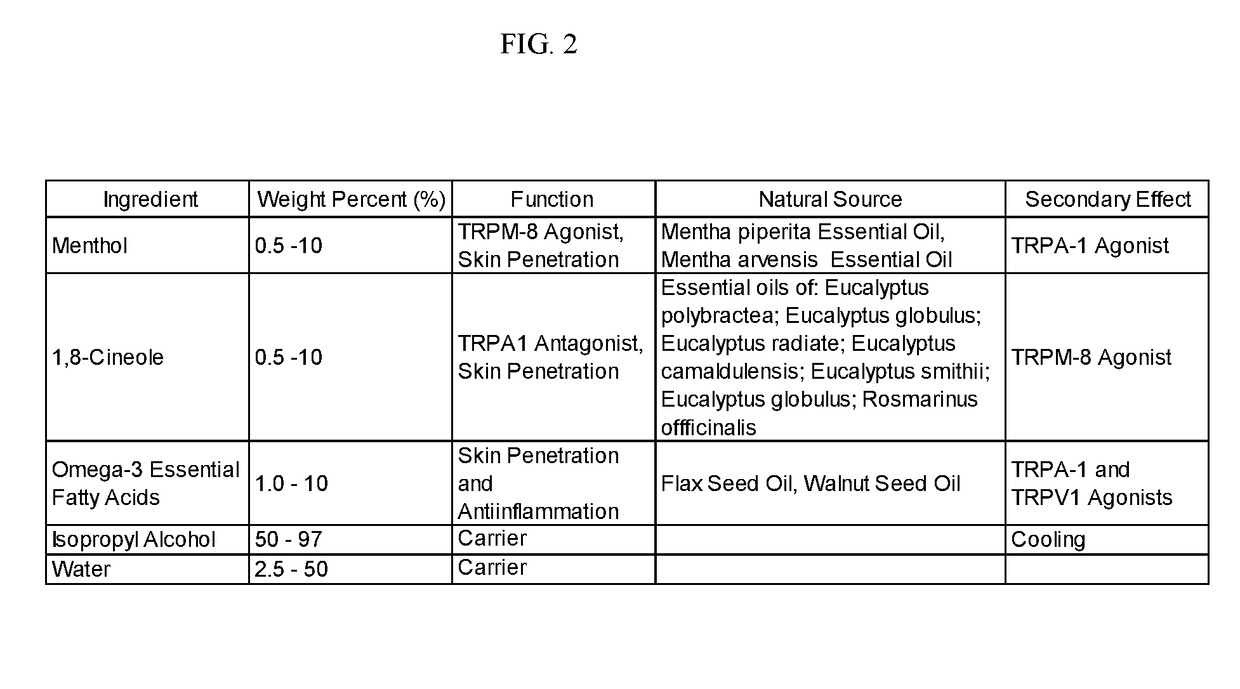
[0239]A method of manufacture of the topical analgesic sprayable liquid containing 1,8-cineole, menthol and Omega-3 fatty acids for the composition presented in Example 2 consists of mixing an amount of water with isopropyl alcohol with the other ingredients in a ratio of alcohol to water, such that a stable single phase homogeneous solution results. Mixing is limited to that required to create a stable single phase homogeneous solution and to minimize volatilization of menthol and 1,8-cineole. Methods of use of the composition of the topical analgesic given in Example 2 include but are not meant to be limited to placing the composition in a spray bottle and spraying onto the skin, placing the composition in a closed aerosol spray vessel under pressure of an inert gas and spraying on the skin and wetting and patch a placing on the skin. The topical analgesic sprayable liquid composition in Example 2 can optionally be made with borneol or a mixture of 1,8-cineole and borneol in the s...
example 3
[0240]A method of manufacture of the topical analgesic gel containing methyl salicylate, 1,8-cineole, menthol and Omega-3 fatty acids for the composition presented in Example 3 consists of mixing an amount sodium polyacrylate with the oil phase components of the compositing to dissolve the sodium polyacrylate then adding an amount of water, such that a stable single phase homogeneous gel results. Mixing is limited to that required to dissolve the sodium polyacrylate with the oil phase components and then to mix the water for a period of time to create the stable single phase homogeneous gel and to minimize volatilization of menthol and 1,8-cineole. Methods of use of the composition of the topical analgesic given in Example 3 include but are not meant to be limited to placing the gel composition in a roll-on bottle then placing the roll-on ball into the roll-on bottle container, placing the gel composition in a squeeze tube container, placing the gel composition in a hand pump bottle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com