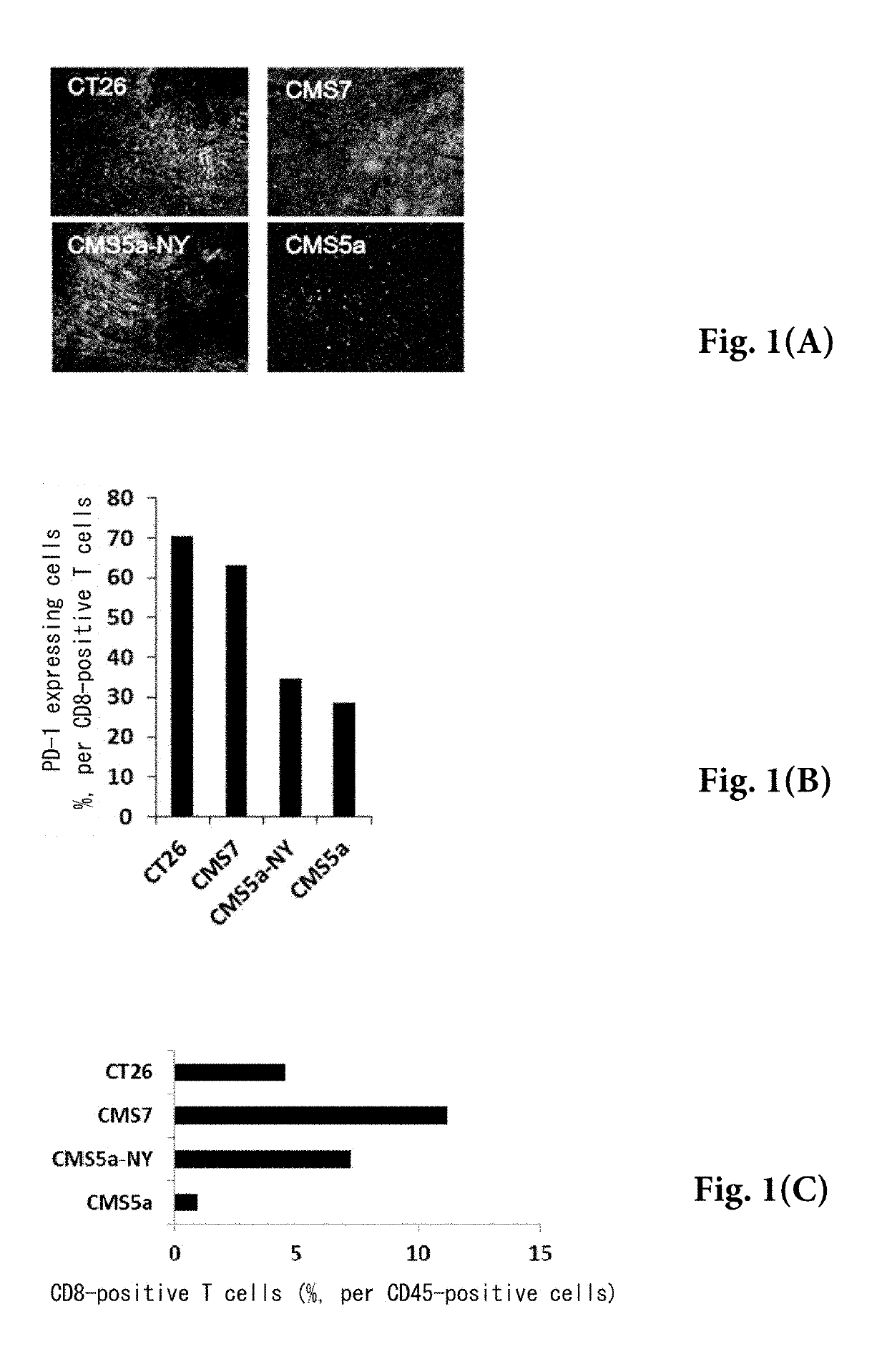
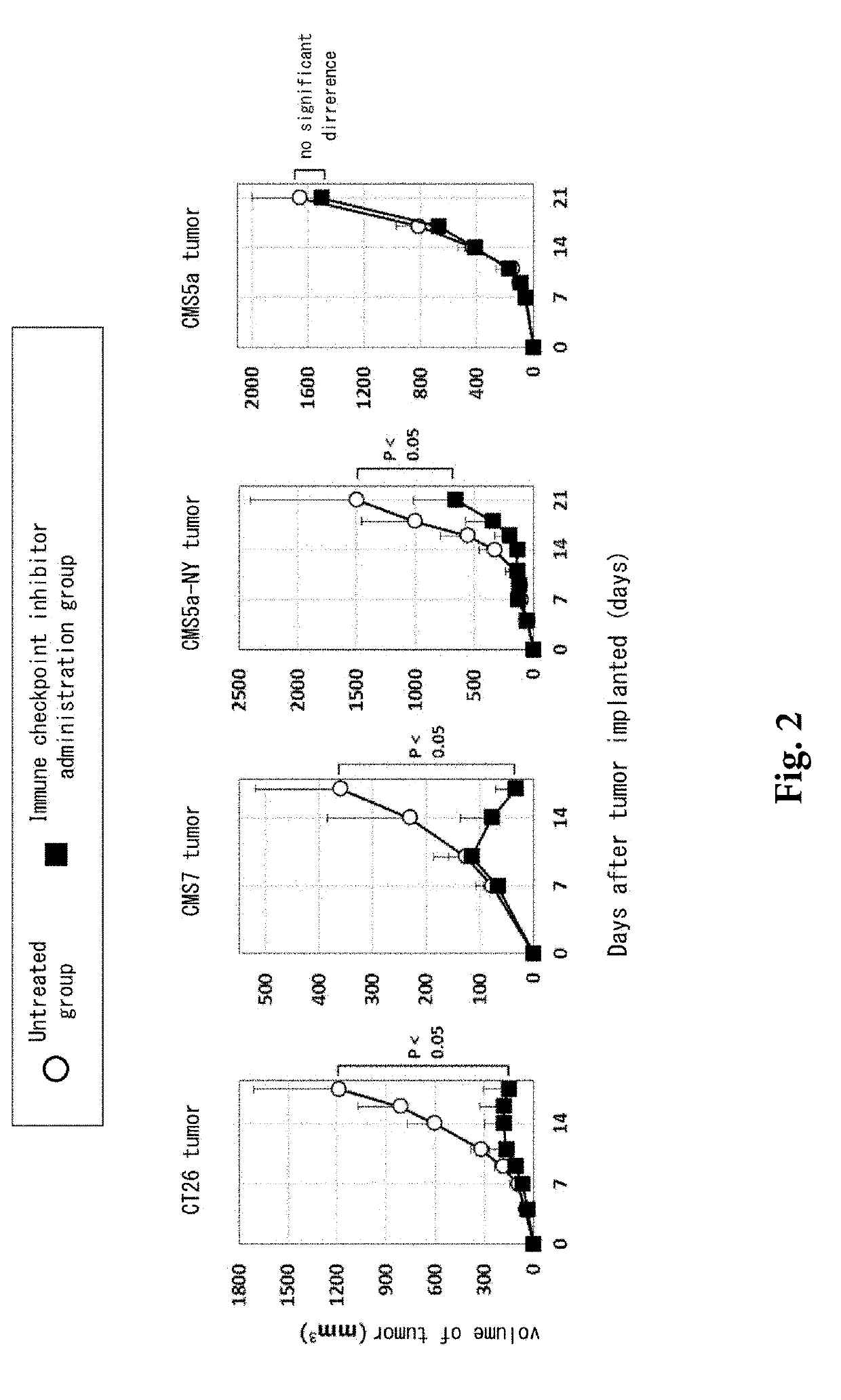
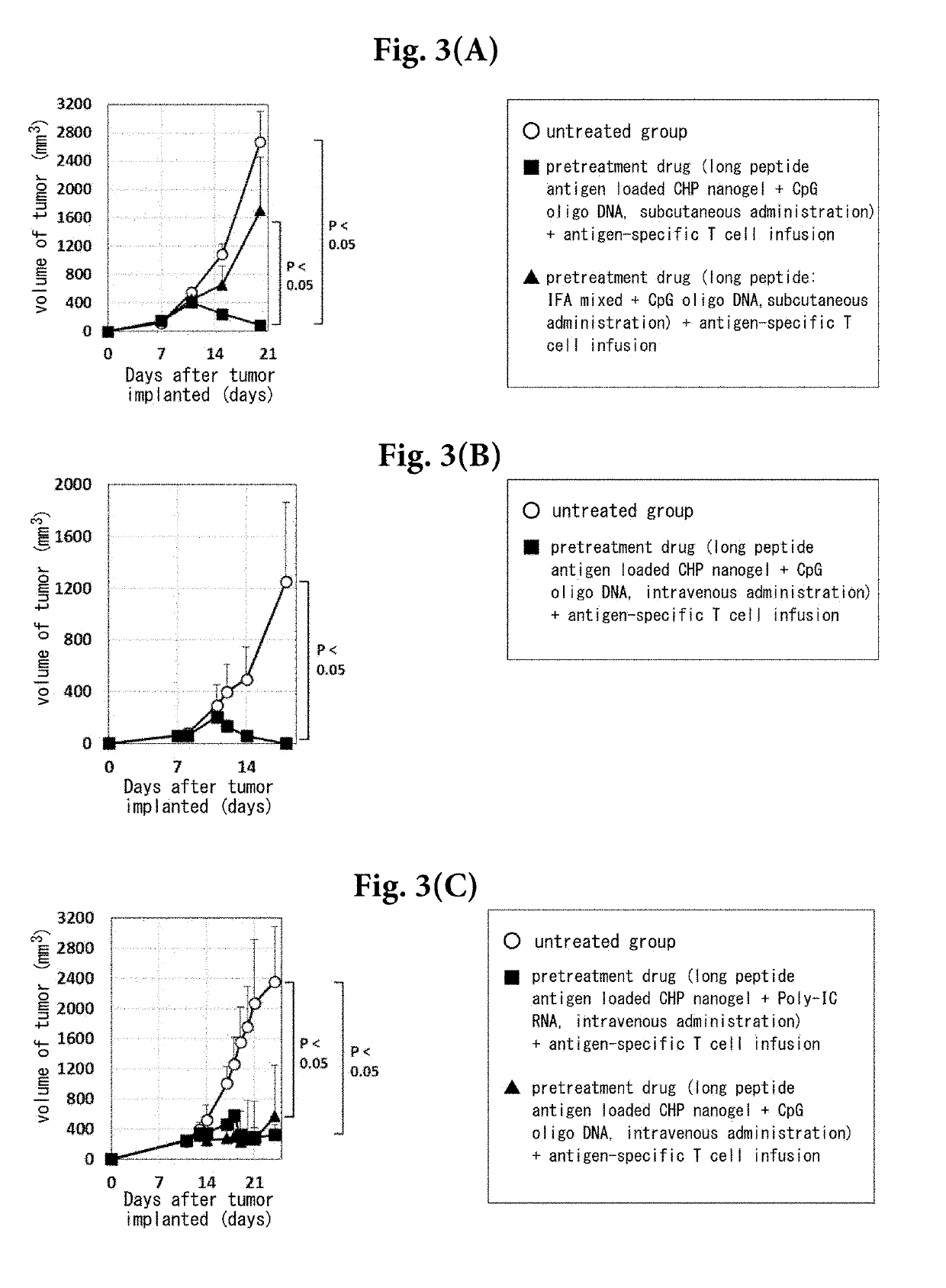
Pretreatment drug for t cell infusion therapy for immune-checkpoint inhibitor-resistant tumor
a technology of immune checkpoint inhibitor and infusion therapy, which is applied in the field of pretreatment drugs, can solve the problems that tumor therapy may be less effective, and achieve the effect of improving the anti-cancer activity of antigen-specific t cell infusions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0064]1. Materials and Methods
[0065]Anti-mouse CD16 / CD32 antibody (clone 93), PE-labeled anti-mouse PD-L1 antibody (clone 9G2), APC-Cy7-labeled anti-CD45 antibody (clone 30-F11), and PE-Cy7-labeled anti-PD-1 antibody (clone 29F.1A12) were purchased from Biolegend. V450-labeled anti-CD8 antibody (clone 53-6.7) was purchased from eBioscience. Fetal bovine serum (FBS) was purchased from Bio-West. RPMI1640 medium (containing 2-mercaptoethanol) was purchased from the Cell Science Institute. Erythrocyte hemolysis solution (0.15 M NH4Cl / 10 mM KHCO3 / 0.1 mM EDTA.Na2 pH 7.2) was prepared at Mie University. Mouse colon cancer CT26 cell line (CRL-2638) was purchased from ATCC and was used as subcultured at Mie University. Mouse fibrosarcoma CMS7 cell line and murine fibrosarcoma CMS5a cell line were obtained from Memorial Sloan-Kettering Cancer Institute and were used as subcultured at Mie University. Human NY-ESO-1 antigen gene was obtained from Memorial Sloan-Kettering Cancer Institute. CMS5a...
example 2
[0071]1. Materials and Methods
[0072]A hybridoma that expresses anti-mouse CTLA-4 antibody (clone 9D9) was obtained from Dr. James P. Allison at the MD Anderson Cancer Center, and antibody was prepared at Mie University. A hybridoma that expresses anti-mouse GITR antibody (clone DTA-1) was obtained from Dr. Shimon Sakaguchi at Osaka University, and antibody was prepared at Mie University. Anti-mouse-PD-1 antibody (clone RMP1-14) was obtained from Dr. Hideo Yagita at Juntendo University. Fetal bovine serum (FBS) was purchased from Bio-West. RPMI1640 medium (containing 2-mercaptoethanol) was purchased from the Cell Science Institute. Mouse colon cancer CT26 cell line (CRL-2638) was purchased from ATCC and was used as subcultured at Mie University. Mouse fibrosarcoma CMS7 cell line and murine fibrosarcoma CMS5a cell line were obtained from Memorial Sloan-Kettering Cancer Institute and were used as subcultured at Mie University. Human NY-ESO-1 antigen gene was obtained from Memorial Sloa...
example 3
[0076]1. Materials and Methods
[0077]Cholesteryl pullulan (abbreviation CHP, trade name CHP-80T) was obtained from NOF Corporation. Incomplete Freund's adjuvant (abbreviation IFA, product number F5506) was purchased from Sigma-Aldrich. Long chain peptide antigen-loaded CHP nanogel was prepared as follows. Long peptides antigens (MEN peptide: SNPARYEFLYYYYYYQYIHSANVLYYYYYYRGPESRLL (SEQ ID NO: 1) and p121 peptide: NDHIAYFLYQILRGLQYIHSANVLHRDLKPSNLLLNT (SEQ ID NO: 2)) were chemically synthesized by Bio-Synthesis and were dissolved in dimethyl sulfoxide (abbreviation DMSO, Nacalai Tesque) at a concentration of 10 mg / mL. CHP was dissolved in phosphate-buffered saline (PBS) containing 6 M urea (Nacalai Tesque) at a concentration of 10 mg / mL. One mL (10 mg) of the long chain peptide antigen solution and 20 mL (200 mg) of the CHP solution were mixed and left overnight with gentle stirring at 4° C. in the dark. The mixture was transferred to a dialysis membrane (molecular weight: 3,500, Therm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com