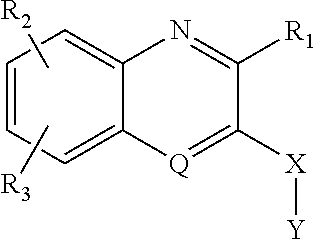
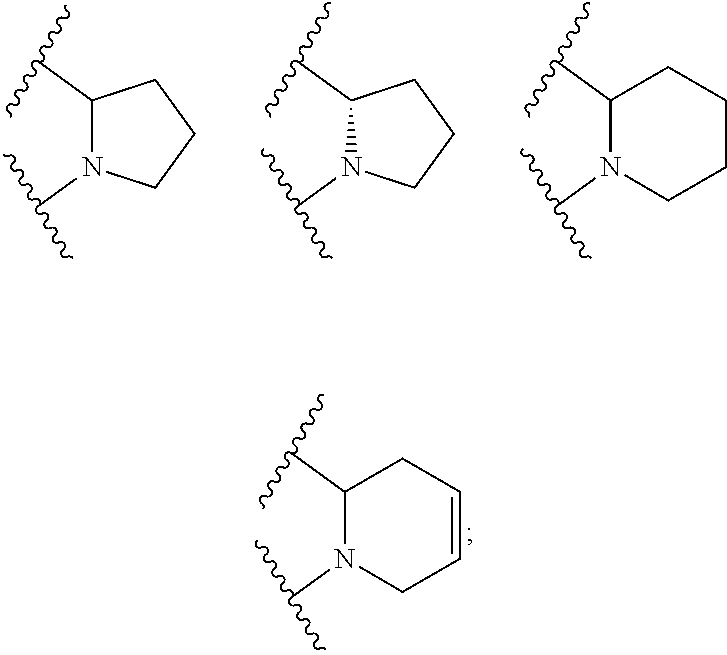
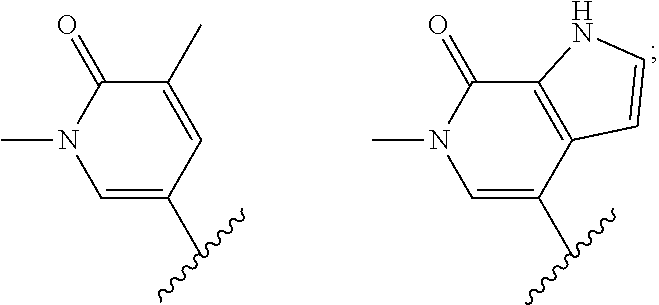
Heterocyclic compounds and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of 4-amino-6-(2-(6-(3,5-dimethylisoxazol-4-yl)-7-methoxy-2-(piperidin-1-yl)quinolin-3-yl)pyrrolidin-1-yl)pyrimidine-5-carbonitrile (1)
[0075]Compound 1 was prepared according to the procedures set forth in steps 1-10 of Scheme 1 below:
Step 1: N-(4-bromo-3-methoxyphenyl)acetamide
[0076]To a solution of 4-bromo-3-methoxyaniline (2.0 g, 9.9 mmol) in CH2Cl2 (25 mL) at 0° C. was added Et3N (4.1 mL, 29.7 mmol), followed by dropwise addition of Ac2O (1.4 mL, 14.9 mmol). The reaction mixture was stirred under argon at 0° C. for 0.5 hour and at room temperature overnight, diluted with CH2Cl2 (25 mL) and water (25 mL). The organic layer was further washed with 1 N acetic acid aqueous solution (25 mL), saturated NaHCO3 aqueous solution (25 mL), brine (25 mL), dried (MgSO4). Evaporation to dryness yielded the title product (2.2 g) as a tan solid. 1H NMR (300 MHz, DMSO-d6) δ 2.04 (s, 3H), 3.80 (s, 3H), 7.10 (dd, J=2.0 Hz and 8.6 Hz, 1H), 7.44 (m, 2H), 10.1 (s, 1H).
Step 2: 6-bromo-2-chloro-7-methox...
example 2
of 4-amino-6-(2-(8-methoxy-1-methyl-7-phenyl-[1,2,4]triazolo[4,3-a]quinolin-4-yl)pyrrolidin-1-yl)pyrimidine-5-carbonitrile (2)
[0087]Compound 2 was prepared according to the procedures set forth in steps 1-4 of Scheme 2 below:
Step 1: tert-butyl 2-(6-bromo-2-hydrazinyl-7-methoxyquinolin-3-yl)pyrrolidine-1-carboxylate
[0088]tert-butyl 2-(6-bromo-2-chloro-7-methoxyquinolin-3-yl)pyrrolidine-1-carboxylate (120 mg, 0.27 mmol) was treated with hydrazine monohydrate (1.0 mL) in EtOH (1 mL) at 110° C. over 24 hours in a sealed tube. The reaction mixture was cooled to room temperature and evaporated to dryness afforded the title compound as a yellow solid. MS (ESI): m / z 437.5, 439.0 (M+H)+; analytical HPLC: 18.8 min.
Step 2: 7-bromo-8-methoxy-1-methyl-4-(pyrrolidin-2-yl)-[1,2,4]triazolo[4,3-a]quinoline
[0089]tert-butyl 2-(6-bromo-2-hydrazinyl-7-methoxyquinolin-3-yl)pyrrolidine-1-carboxylate (75 mg, 0.19 mmol) in acetic acid (3 mL) was stirred at 100° C. for 3 hours. Evaporation to dryness and pur...
example 3
of 4-amino-6-(2-(2-(3,5-dimethylisoxazol-4-yl)-8-methylquinolin-3-yl)pyrrolidin-1-yl)pyrimidine-5-carbonitrile (3)
[0092]Compound 3 was prepared according to the procedures set forth in steps 1-3 of Scheme 3 below:
Step 1: tert-butyl 2-(2-(3,5-dimethylisoxazol-4-yl)-8-methylquinolin-3-yl)pyrrolidine-1-carboxylate
[0093]A mixture of tert-butyl 2-(2-chloro-8-methylquinolin-3-yl)pyrrolidine-1-carboxylate (30 mg, 0.087 mmol; that was prepared according to the procedures disclosed in U.S. Pat. No. 9,637,488), 3,5-dimethylisoxazol-4-ylboronic acid (13.7 mg, 0.097 mmol), and Pd(dppf)2Cl2.CH2Cl2 (3.5 mg), Cs2CO3 (100 mg) in water (0.27 mL) and 1,2-domethoxyethane (0.81 mL) was purged with argon for 5 min and then stirred at 90° C. overnight in a sealed tube. The reaction mixture was cooled to room temperature, and evaporated to dryness. Purification by silica gel chromatography using EtOAc / hexane (12.5% to 25%) afforded the title compound (6.8 mg) as a white solid. MS (ESI): m / z 480.4 (M+H)+.
S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com