Ascorbic acid and quinone compounds for treating chagas disease
a technology of ascorbic acid and quinone, which is applied in the direction of antiparasitic agents, organic active ingredients, drug compositions, etc., can solve the problems of high toxicity, variable efficacy of benznidazole and nifurtimox,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Trypanocidal Effect of Vitamins C and K3
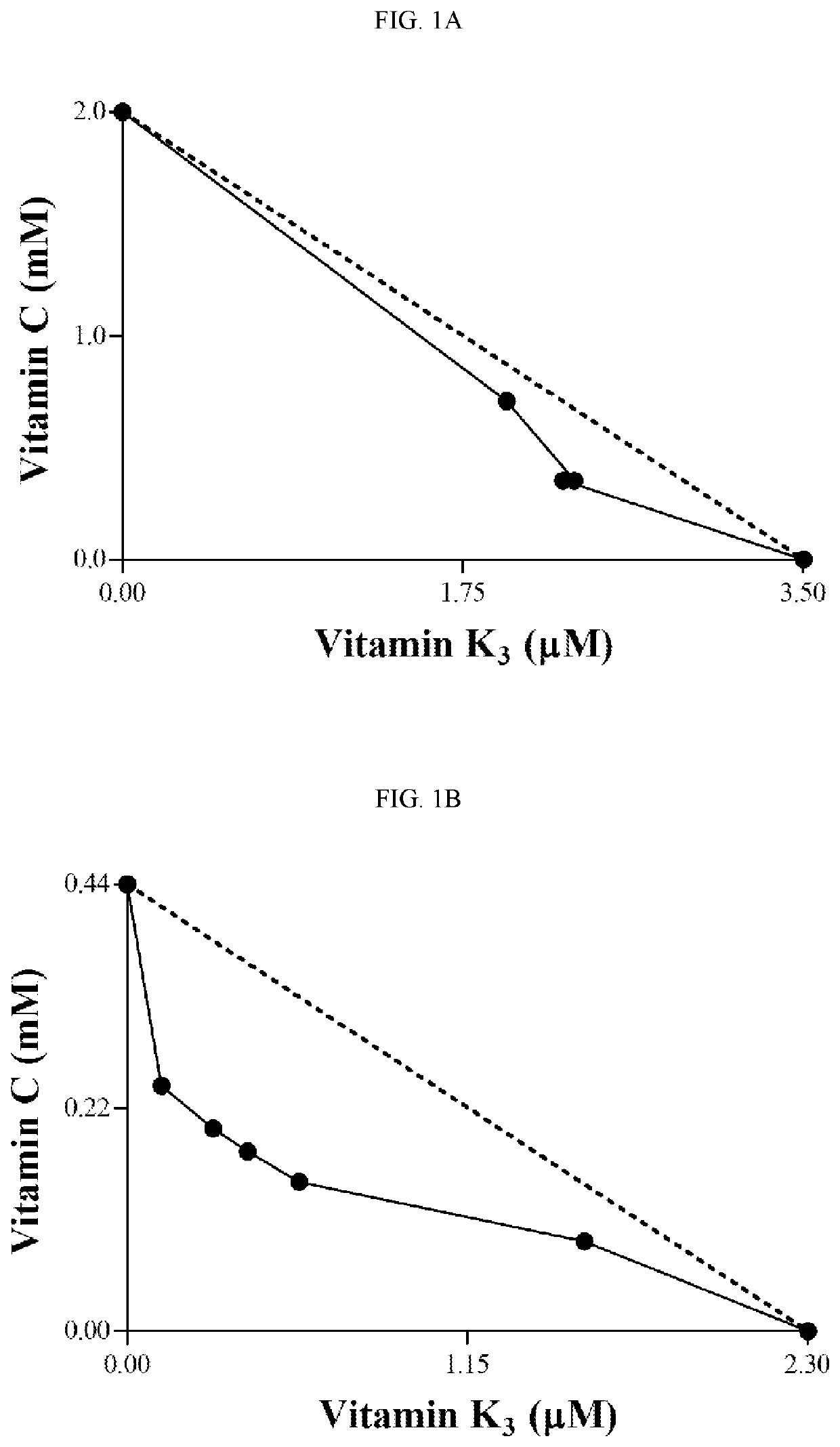
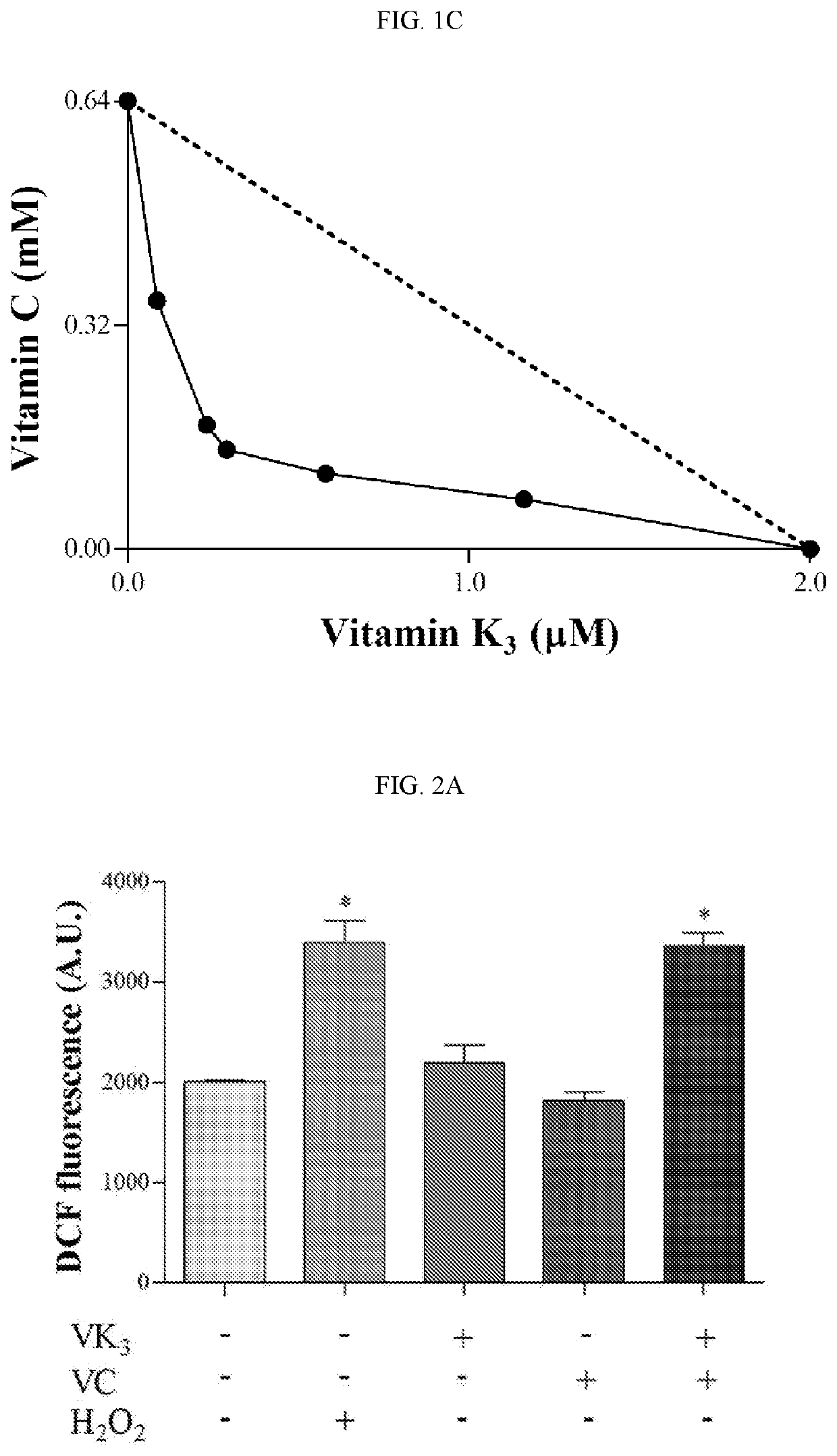
[0207]To evaluate the trypanocidal effect of the VC and VK3 combination on epimastigotes, trypomastigotes, and amastigotes, the combination Index method as described by Chou and Talalay was applied. Chou et al., Adv. Enzyme Regul. 1984, 22, 27-55.
[0208]Epimastigote forms (1×106 parasites / mL) in the exponential growth phase were resuspended in LIT medium supplemented with 10% FBS. The vitamins were added to the cell suspension, alone or in combination (0.35-2.84 mM VC and 1.0-9.0 μM VK3), in 24-well plates and incubated at 28° C. The number of epimastigote forms was determined by counting in a Neubauer hemocytometer after 96 hrs.
[0209]To evaluate activity against trypomastigote forms, parasites were obtained from the supernatant of infected LLCMK2 cells. Trypomastigote forms (1×107 parasites / mL) were resuspended in the presence of DMEM supplemented with 10% FBS and different concentrations of both vitamins, alone or in combination (0.09-1.42 m...
example 2
[0213]The morphological and ultrastructural effect of vitamins C and K3
[0214]For scanning electron microscopy (SEM), epimastigote forms (1×106 parasites / mL) were treated with 0.61 mM VC and 1.90 μM VK3, alone or in combination, for 72 hrs at 28° C. Trypomastigote forms (1×107 parasites / mL) were treated with 0.20 mM VC and 0.35 μM VK3, alone or in combination, for 24 hrs at 37° C. in a 5% CO2 atmosphere. Intracellular amastigotes were treated with 0.18 mM VC and 0.30 μM VK3, alone or in combination, for 24 hrs at 37° C. in a 5% CO2 atmosphere. After incubation, the parasites were harvested, washed twice in PBS, and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4° C. The parasites were then placed on a glass support that was covered with poly-L-lysine, dehydrated in an ascending series of ethanol, critical-point dried with CO2, coated with gold, and observed in a Shimadzu SS-550 scanning electron microscope. For intracellular amastigotes, we used the fracture ta...
example 3
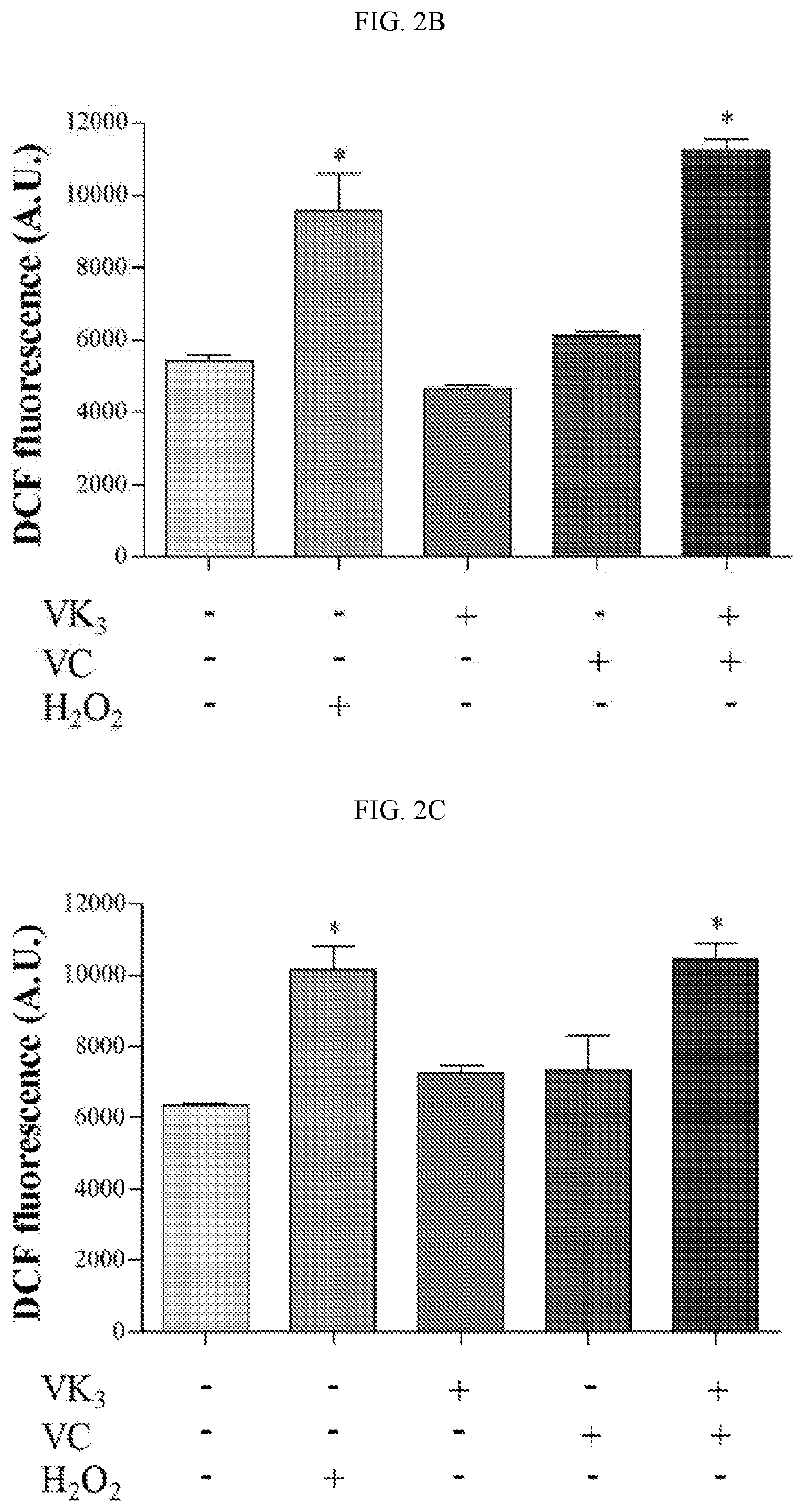
The Effect of Vitamins C and K3 on the Generation of Total ROS and NO
[0218]The production of total reactive oxygen species (total ROS) was evaluated in parasitic forms after exposure to VC and VK3 using the probe H2DCFDA. Epimastigote forms (1×106 parasites / mL) were evaluated after exposure to 0.61 mM VC and 1.90 μM VK3, alone and in combination, for 24 hrs at 28° C. Trypomastigote forms (1×107 parasites / mL) were evaluated after exposure to 0.20 mM VC and 0.35 μM VK3, alone and in combination, for 24 hrs at 37° C. in a 5% CO2 atmosphere. Amastigote forms (1×107 parasites / mL) were evaluated after exposure to 0.18 mM VC and 0.30 μM VK3, alone and in combination, for 24 hrs at 37° C. in a 5% CO2 atmosphere. Hydrogen peroxide (H2O2; 20.0 μM) was used as a positive control. Afterward, the parasites were centrifuged, washed, and resuspended in PBS. Parasites were loaded with 10.0 μM of the permeant probe H2DCFDA in the dark for 45 min. Total ROS were measured as an increase in fluorescenc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com