Catalyst for hydrosilylation reaction, hydrogenation reaction, and hydrosilane reduction reaction
a hydrogenation reaction and catalyst technology, applied in the field of catalysts, can solve the problems of reducing the selectivity of -adducts, affecting the catalytic activity of the reaction, and the side reaction of internal rearrangement of olefin, and achieve high catalytic activity, high thermal stability, and high stability in the air of the complex
Inactive Publication Date: 2020-01-02
KYUSHU UNIV +1
View PDF0 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
[0137]A catalyst for reaction made of an iron-, cobalt-, or nickel-isocyanide complex (hereinafter, simply abbreviated as an isocyanide complex) of the present invention does not have a carbonyl ligand highly toxic to human bodies, and has high thermal stability and high stability in air of the complex.
[0138]When the isocyanide complex of the present invention is used as a catalyst to perform hydrosilylation reaction between an aliphatic unsaturated bond-containing compound and a silane or a (poly)siloxane having a Si—H group, addition reaction can be made under conditions of room temperature to less than or equal to 100° C. In particular, also addition reaction with an industrially useful (poly)siloxane, a trialkoxysilane, or a dialkoxysilane progresses well. A known literature shows that, in the same reaction, both addition reaction on an unsaturated bond and reaction that produces an unsaturated bond-containing compound due to dehydrogenative silylation reaction progress simultaneously in many cases; in contrast, when the catalyst of the present invention is used, addition reaction on an unsaturated bond progresses selectively.
[0139]In addition, in reaction with an internal olefin, which has been difficult for conventional catalysts, a product of addition reaction accompanied by movement of an unsaturated bond to an end can be obtained.
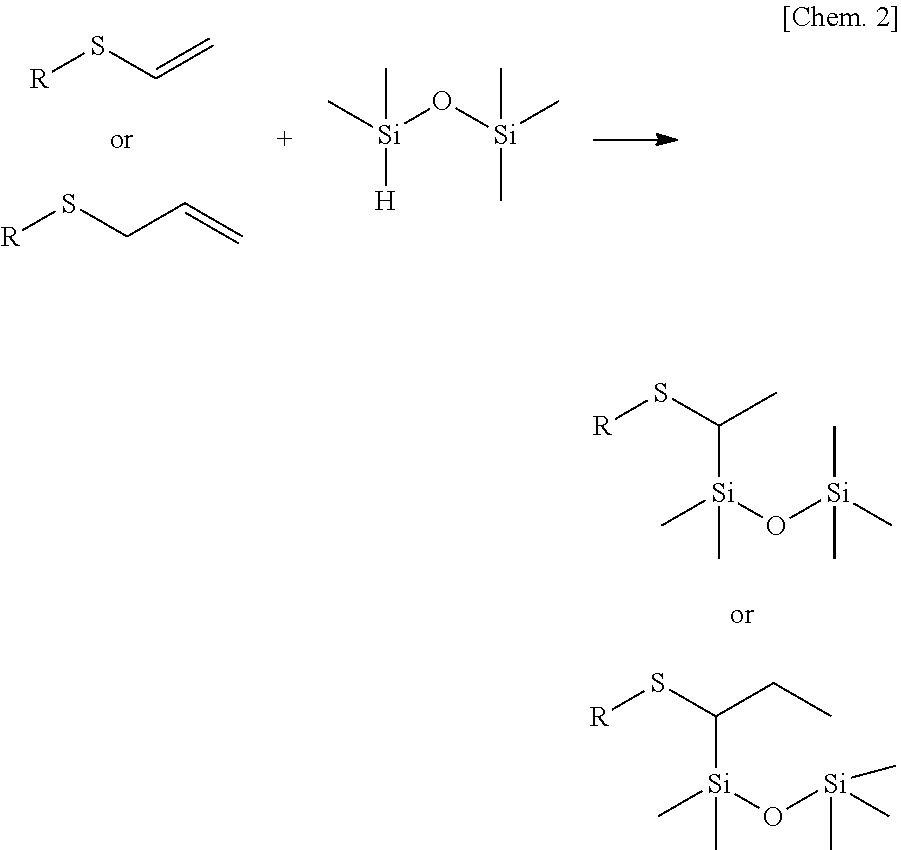
[0140]Further, by using the catalyst of the present invention, in hydrosilylation reaction of an alkenyl sulfide or the like, an addition product in which Si is bonded to carbon adjacent to the sulfur element is obtained selectively.
[0141]Further, the catalyst of the present invention has high catalytic activity on hydrogenation reaction of an aliphatic unsaturated bond-containing compound, and the reaction progresses under mild conditions.
[0142]Further, in hydrosilane reduction reaction on a carbon-oxygen unsaturated bond or a carbon-nitrogen unsaturated bond using the catalyst of the present invention, a carbonyl compound such as an amide compound, a ketone compound, or an amide compound, or a nitrile compound, and an easy-to-handle silane or (poly)siloxane having a Si—H group are reacted together, and the desired compound can be obtained with a high yield.
Problems solved by technology
While several problems arise with reaction in the presence of Pt compounds as the catalyst, one problem is that upon addition of a Si—H functional compound to terminal olefin, a side reaction due to internal rearrangement of olefin takes place.
Another problem is that the selectivity of α- and β-adducts is low depending on the type of olefin.
The most serious problem is that all the center metals Pt, Pd and Rh are quite expensive noble metal elements.
Although PhSiH3 and Ph2SiH2 add to olefins, more useful trialkylsilanes, alkoxysilanes and siloxanes have poor addition reactivity to olefins.
This method needs a catalyst synthesis, including first synthesizing a terpyridine-iron complex as a catalyst precursor and introducing a bistrimethylsilylmethyl group therein at a low temperature, which is not easy.
However, at the time of the synthesis of this complex, there are points at issue such as using Na amalgam, which consists of water-sensitive sodium and highly toxic mercury and needs care in handling (or using water-sensitive NaBEt3H), and the storage requiring the conditions of being at low temperature in an inert-gas nitrogen atmosphere because of the low stability of the complex compound itself.
However, a reducing agent (NaBHEt3) is needed, and dihydrodiphenylsilane is not a reaction substrate having high industrial value.
Also an example of reaction by a cobalt-carbonyl complex (Co2(CO)8 or the like) is reported (Non-Patent Documents 10 to 15); however, this is not satisfactory in terms of reaction yield or reaction molar ratio, and the complex has highly toxic carbon monoxide and the handling and storage of the complex require the conditions of being in an inert gas atmosphere and at low temperature.
Because of low stability, these complex compounds require an inert gas atmosphere and a low temperature for handling and storage.
However, the catalytic activity is lower than in other substrates, and the selectivity of the addition reaction product is low.
The example using a Rh catalyst reports that an addition product in which Si is bonded to carbon adjacent to the sulfur element is obtained selectively (Non-Patent Document 25); however, the catalytic activity is low, and the selectivity of the adduct is low.
For example, a catalyst having a phosphine ligand (Non-Patent Document 26) lacks in selectivity and requires careful handling and storage.
Like the above-cited Non-Patent Documents 6 to 8, there is an industrial difficulty of synthesis of a catalyst precursor or synthesis of the complex catalyst from the precursor.
Likewise, the yield of the desired product is less than satisfactory.
However, reactivity is empirically demonstrated with respect to only platinum, palladium, rhodium and iridium which are expensive metal elements.
Thus the method is not regarded cost effective.
In addition, the metal catalysts coordinated with carbene require careful handling because the complex compounds have low storage stability.
However, thermal reaction requires conditions of high temperature and high pressure (180° C., 28 atmospheres); in contrast, photoreaction progresses at room temperature; but both have low turnover numbers (TON), which number indicates the number of revolutions of the catalyst, and cannot be said to have sufficient activity.
Also an example of an iron catalyst having a 1,2-bis(dimethylsilyl)benzene ligand is reported (Non-Patent Document 37); in this example, although reaction progresses at room temperature under normal pressure, the synthesis of the catalyst is not easy.
Further, an iron catalyst having a 2,6-bis(arylimidazol-2-ylidene)pyridine ligand is reported (Non-Patent Document 38); however, both have points at issue such as safety at the time of synthesis and the stability of the compound, similarly to the Fe complex having a bis(imino)pyridine ligand mentioned above.
Also a cobalt catalyst having a phosphorus-based compound as a ligand is reported (Non-Patent Document 39); in this system, although reaction progresses at room temperature under normal pressure, the synthesis of the catalyst is not easy.
A cobalt catalyst having a bis(mesitylbenzimidazol-2-ylidene)phenyl ligand is reported (Non-Patent Document 40); however, this has points at issue such as safety at the time of synthesis and the stability of the compound, similarly to the Fe complex having a 2,6-bis(arylimidazol-2-ylidene)pyridine ligand mentioned above, and furthermore the catalytic activity cannot be said to be sufficient, in view of the TON being 50.
However, these reducing agents are ignitable, water-sensitive substances, and are therefore less easy to handle.
A large number of methods in which a hydrosilane compound or methyl hydrogen polysiloxane, which is stable and easy to handle in air, is used as a reducing agent are reported; however, the reaction requires the addition of a strong acid or a Lewis acid, or the use of an expensive noble metal catalyst.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
examples 2 and 3
[0257]Reaction was performed in a similar manner to Example 1 except that, in place of Co2(CNtBu)8, the cobalt-isocyanide complexes written in Table 1 (each 0.005 mmol) were used as catalysts. The results are shown in Table 1 below.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Login to view more
Abstract
Provided is a catalyst which comprises a compound represented by formula (1) and which exhibits activity for at least one type of reaction selected from among hydrosilylation reaction or hydrogenation reaction with respect to an aliphatic unsaturated bond and hydrosilane reduction reaction with respect to a carbon-oxygen unsaturated bond or a carbon-nitrogen unsaturated bond. Formula (1): Mn(Lm) {M represents Fe, Co, or Ni having an oxidation number of 0, L represents an isocyanide ligand represented by formula (2), n denotes an integer of 1-8, and m denotes an integer of 2-12. Formula (2): (CN)x—R1 (R1 represents a mono- to trivalent-organic group having 1-30 carbon atoms, optionally being substituted by a halogen atom, and optionally having interposed therein one or more atoms selected from among O, N, S, and Si; and x denotes an integer of 1-3)}.
Description
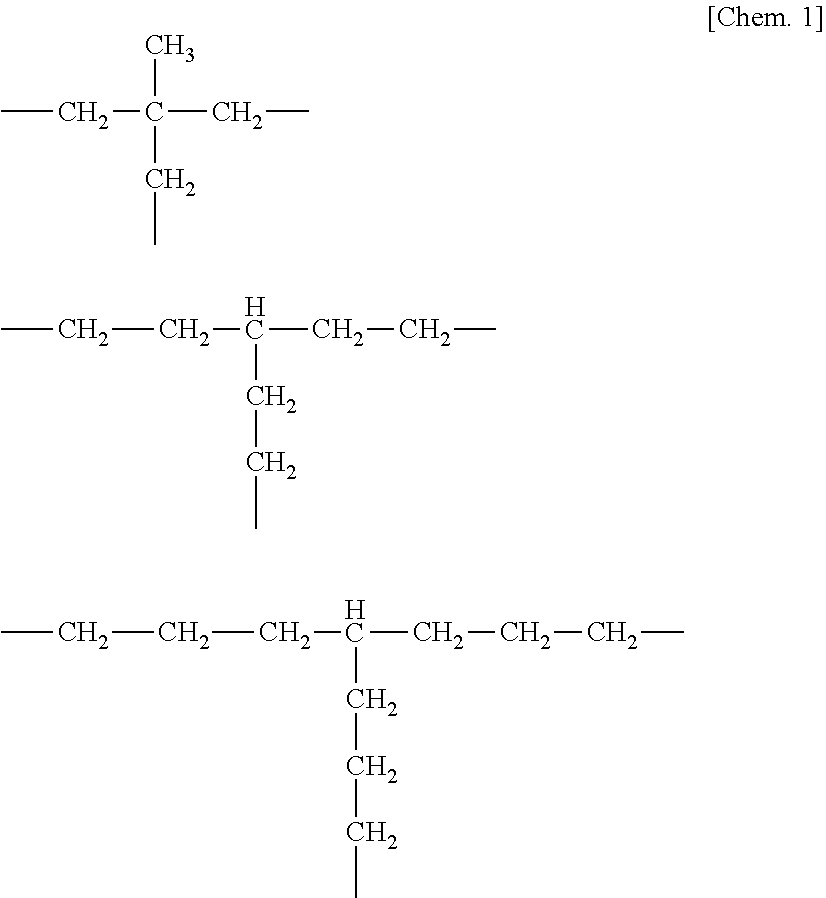
TECHNICAL FIELD[0001]The present invention relates to a catalyst made of a prescribed metal-isocyanide complex, and relates more specifically to a catalyst having activity in at least one reaction selected from hydrosilylation reaction or hydrogenation reaction on an aliphatic unsaturated bond and hydrosilane reduction reaction on a carbon-oxygen unsaturated bond or a carbon-nitrogen unsaturated bond.BACKGROUND ART[0002]Hydrosilylation reaction which is addition of a Si—H functional compound to a compound having a carbon-carbon double bond or triple bond is a useful method for the synthesis of organosilicon compounds and an industrially important synthesis reaction.[0003]As the catalyst for hydrosilylation reaction, Pt, Pd and Rh compounds are known. Among others, Pt compounds as typified by Speier's catalyst and Karstedt's catalyst are most commonly used.[0004]While several problems arise with reaction in the presence of Pt compounds as the catalyst, one problem is that upon additi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more Application Information
Patent Timeline
 Login to view more
Login to view more Patent Type & Authority Applications(United States)
IPC IPC(8): B01J31/22C07F7/10C07F7/08
CPCC07F7/10C07F7/0829B01J2231/323B01J2531/847B01J2531/845B01J2531/842C07B47/00B01J31/2282B01J31/22C07B61/00C07C5/03C07C9/15C07C15/073C07C209/50C07C211/27C07F7/08C08G77/08B01J2231/645B01J2531/0219B01J2531/0222C07F7/0879C07F7/21B01J2231/60
Inventor NAGASHIMA, HIDEOSANAGAWA, ATSUSHIKAWABATA, SHOMANODA, DAISUKESAKUTA, KOJI
Owner KYUSHU UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap