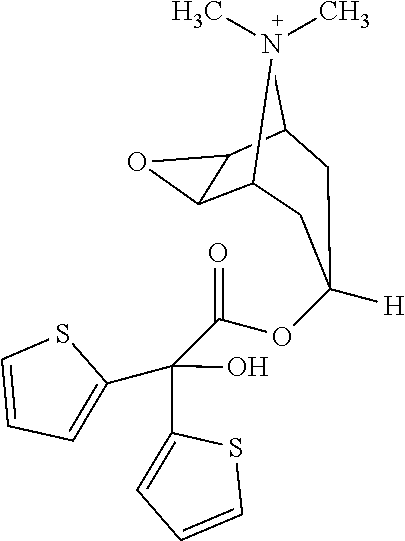
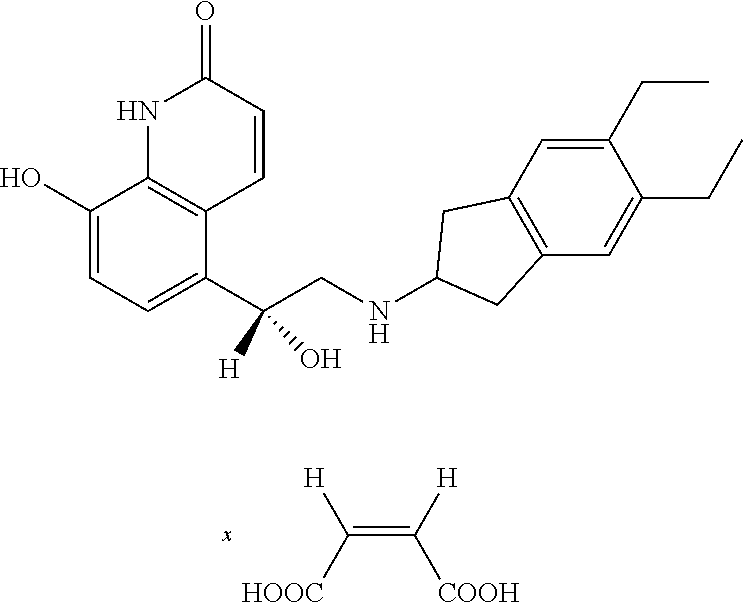
Nebulization composition comprising tiotropium and indacaterol
a technology of tiotropium and indacaterol, which is applied in the direction of organic active ingredients, respiratory disorders, pharmaceutical non-active ingredients, etc., can solve the problems of difficult patient breathing, clogging of airways, life-threatening, etc., and achieves convenient, fast and reliable treatment, the effect of improving user compliance and/or quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0157]
Amount in %w / v#12345678Tiotropium0.0001-0.008bromideIndacaterol0.0003-0.064maleateSodium0.90.90.9 0.90.9 0.9 0.90.9chlorideCitric acid1.9231.9230.9613.845 1.923 1.923——monohydrateTrisodium0.250.250.1250.50.250.25——citratedihydrateEdetate0.01-0.02—————0.01-0.020.01-0.02disodiumHCl———————q.s.Polysorbate 20————0.02———Polysorbate 80—————0.02——Sodium—————— 0.94—dihydrogenphosphatedihydrateAnhydrous—————— 0.175—disodiumhydrogenphosphateWater forq.s.q.s.q.s.q.s.q.s.q.s.q.s.q.s.injection
Manufacturing Process
[0158]1. Dissolve sodium chloride, edetate disodium, citric acid monohydrate, trisodium citrate dihydrate or sodium dihydrogen phosphate dihydrate, and anhydrous disodium hydrogen phosphate in water for injection with stirring. Add hydrochloric acid to adjust the pH (composition #8).[0159]2. Check the clarity of the solution.[0160]3. Add indacaterol maleate (optionally dissolved in Polysorbate 20 or Polysorbate 80) to the product of step 1 with stirring.[0161]4. Sonicate until a cl...
example 2
[0165]
Amount in μg / 2 ml#1234Tiotropium 5-40 μg / 2 mlbromideIndacaterol10-300 μg / 2 mlmaleateSodium chloride16000160001600016000Tartaric acid5000500050005000Monosodium Citrate2000200020002000Edetate disodium200—200—Benzalkonium Chloride400400——Water for injectionq.s. 2 mlq.s. 2 mlq.s. 2 mlq.s. 2 ml
Manufacturing Process
[0166]1. Dissolve batch quantities of sodium chloride, edetate disodium, tartaric acid and monosodium citrate in water for injection with stirring. Check the clarity of the solution.[0167]2. Add indacaterol maleate to the product of Step 1.[0168]3. Stir / homogenize / sonicate until a clear solution is obtained.[0169]4. Add tiotropium bromide to the product of Step 3 with stirring.[0170]5. Add benzalkonium chloride (BKC) to the product of Step 4 and mix. Make up the volume with water for injection.[0171]6. Filter the product of Step 5 through a sterilizing grade filter.[0172]7. Fill the product of Step 6 in LDPE smartules / HDPE, PP, LDPE bottles / amber glass bottles / amber glass...
example 3
[0173]
Amount in μg / 2 ml#1234Tiotropium 5-40 μg / 2 mlbromideIndacaterol10- 300 μg / 2 mlmaleateSodium chloride16000160001600016000Tartaric acid5000500050005000Sodium tartrate2000200020002000dihydrateEdetate disodium200—200—Benzalkonium chloride400400——Water for injectionq.s. 2 mlq.s. 2 mlq.s. 2 mlq.s. 2 ml
Manufacturing Process
[0174]1. Dissolve batch quantities of sodium chloride, edetate disodium, tartaric acid and sodium tartrate dihydrate in water for injection with stirring. Check the clarity of the solution.[0175]2. Add indacaterol maleate to the product of Step 1.[0176]3. Stir / homogenize / sonicate until a clear solution is obtained.[0177]4. Add tiotropium bromide to the product of Step 3 with stirring.[0178]5. Add benzalkonium chloride (BKC) to the product of Step 4 and mix. Make up the volume with water for injection.[0179]6. Filter the product of Step 5 solution through sterilizing grade filter.[0180]7. Fill the product of Step 6 in LDPE smartules / HDPE, PP, LDPE bottles / amber glas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com