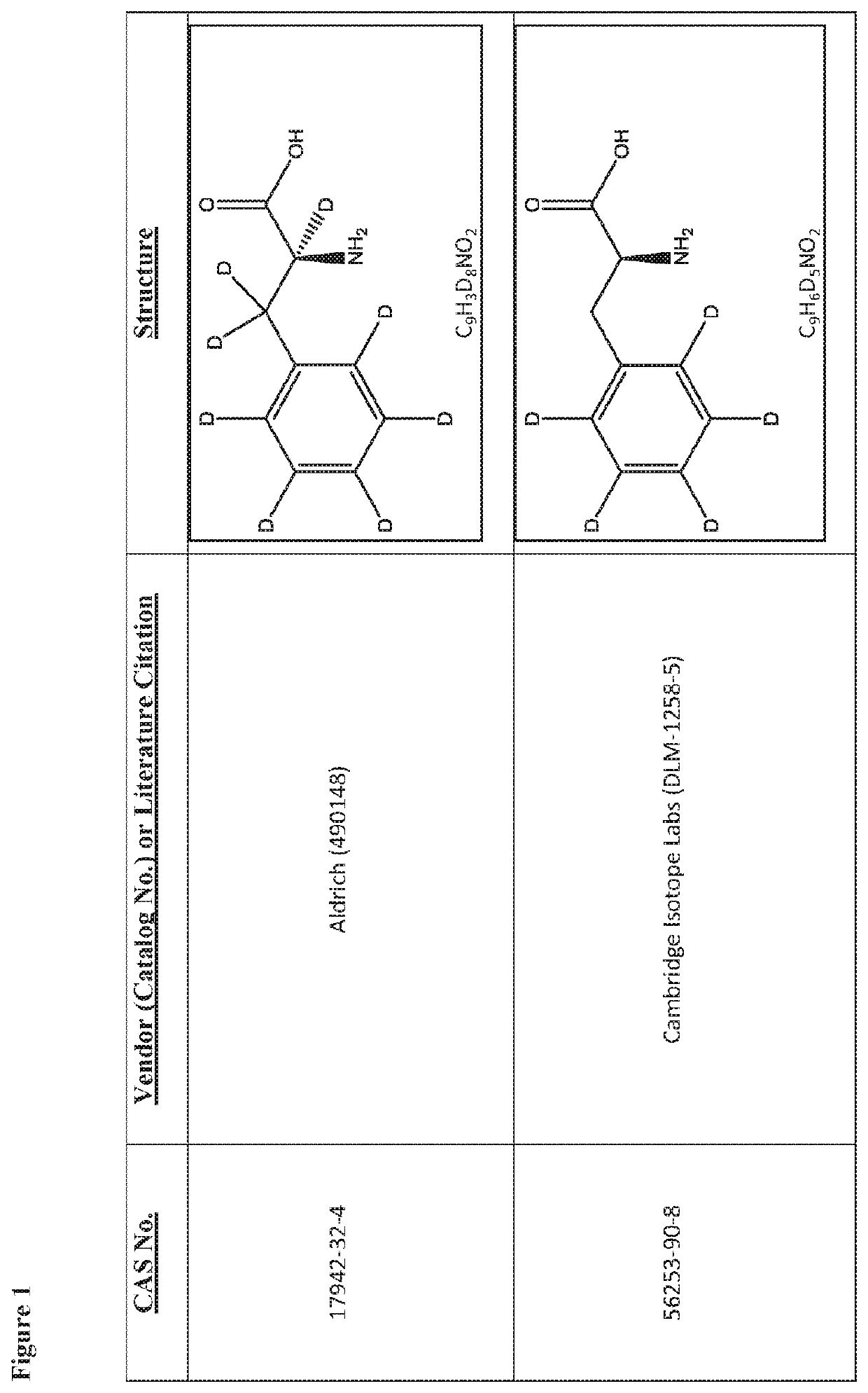
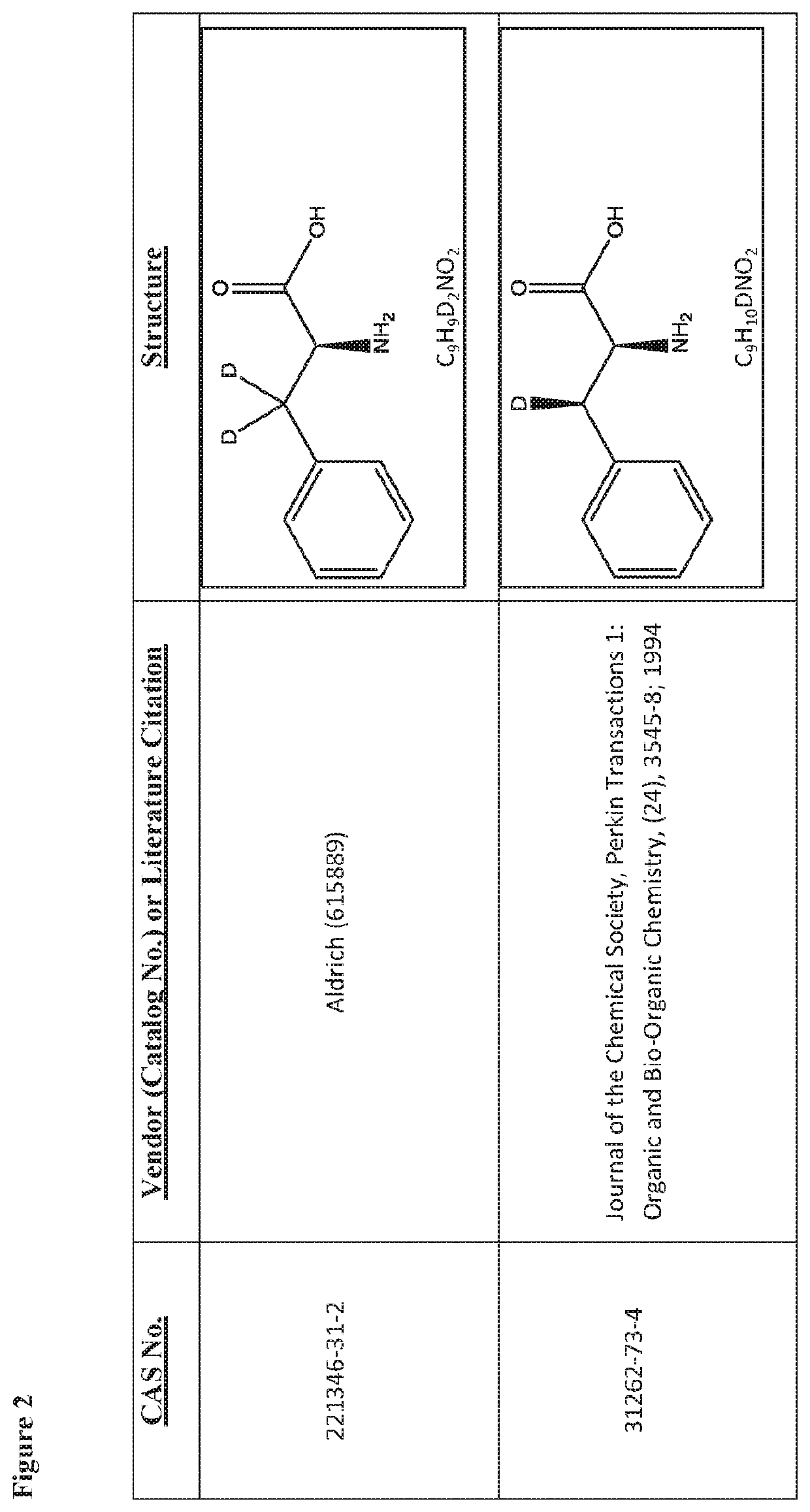
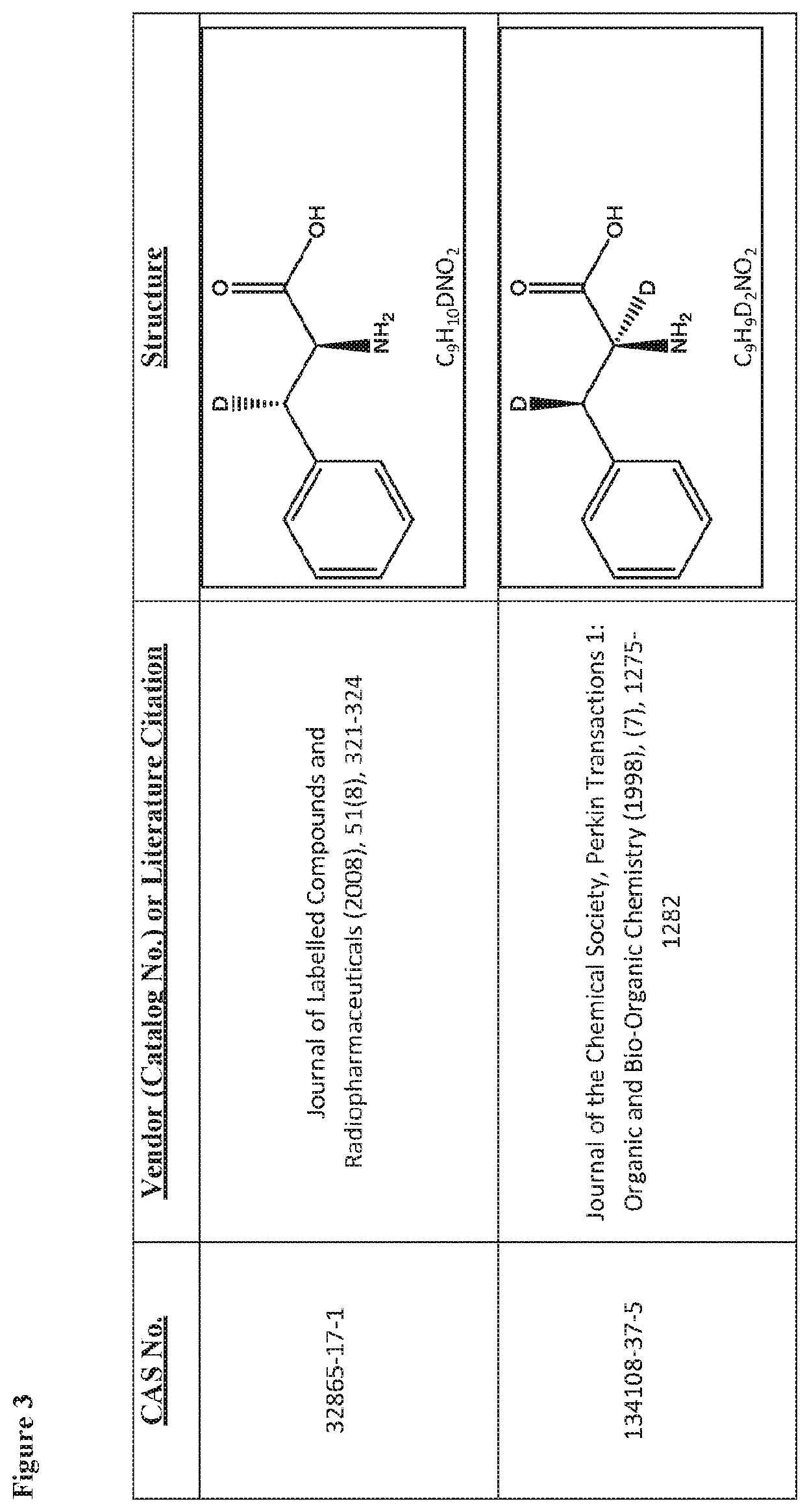
Deuterated tetrapeptides that target mitochondria
a technology of mitochondria and deuterated tetrapeptides, which is applied in the direction of peptides, drug compositions, peptides/protein ingredients, etc., can solve the problems of poor absorption, distribution, metabolism and/or excretion (adme) properties, and many current medicines suffer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Phe-D-Arg-Phe-(3,3,4,4,5,5,6,6-octadeuterium)-Lys-NH2
[0264]
Step 1. Synthesis of (N2-Cbz-N6-Boc-3,3,4,4,5,5,6,6-d8)-Lys-NH2
[0265]
[0266]To a stirring solution of (N2-Cbz-N6-Boc-3,3,4,4,5,5,6,6-d8)-L-Lys-OH (A, 1717 mg, 4.42 mmol) in dry DMF (22 mL) was added carbonyldiimidazole (932 mg, 5.75 mmol) in one portion. The mixture stirred for 1.5 h at rt then aqueous ammonia (0.45 mL, 6.63 mmol, 14.8 M) was added dropwise. After stirring an additional 3 h, no starting material remained (LC / MS). The mixture was concentrated. The resulting residue was partitioned between DCM (130 mL) and sat. NaHCO3 (50 mL). The organic layer was washed with sat. NaHCO3 (2×50 mL), water (50 mL), 5% citric acid (2×50 mL), then brine (50 mL). The organic layer was then dried over anh. Na2SO4, and concentrated to yield product (1599 mg, 93%) as white solid. Used further without additional purification. 1H NMR (300 MHz, CD3OD-d4) δ: 7.43-7.24 (m, 5H), 5.16-5.01 (m, 2H), 4.06 (s, 1H), 1.42 (s, 9H).
Step 2. Synth...
example 2
of Phe-D-Arg-Phe-(4,4,5,5-d4)-Lys-NH2 Trifluoroacetate
[0273]
Step 1. Synthesis of (N2-benzyloxycarboxyl-N6-tert-butoxycarboxyl-4,4,5,5-d4)-Lys-NH2
[0274]
[0275]To a stirring solution of (N2-Cbz-N6-Boc-4,4,5,5-d4)-Lys-OH (F, 384 mg, 1.0 mmol) in dry DMF (5 mL) was added carbonyldiimidazole (162 mg, 1.3 mmol) in one portion. The mixture stirred for 1.5 h at rt then aqueous ammonia (0.10 mL, 1.5 mmol, 14.8 M) was added drop-wise. After stirring an additional 3 h, no starting material remained (LC / MS). The mixture was concentrated. The resulting residue was partitioned between DCM (30 mL) and sat. NaHCO3 (12 mL). The organic layer was washed with sat. NaHCO3 (2×12 mL), water (12 mL), 5% citric acid (2×12 mL), then brine (12 mL). The organic layer was then dried over anh. Na2SO4, and concentrated (TLC, DCM-MeOH (10:1) Rf(PR) 0.5). (N2-Cbz-N6-Boc-4,4,5,5-d4)-Lys-OH (376 mg, 98%), which was used without further purification. 1H NMR (300 MHz, CD3OD-d4) δ: 7.43-7.20 (m, 5H), 5.17-5.00 (m, 2H), ...
example 3
of Phe-D-Arg-Phe-(2,5,5-d3)-Lys-NH2 Trifluoroacetate
[0282]
Step 1: Synthesis of (N6-Boc-2,5,5-d3)-Lys
[0283]
[0284]L-(2,5,5-d3)-Lysine hydrochloride (J, 1.30 mmol, 241 mg) was dissolved in 2 M NaHCO3 (1.30 mL, 2.6 mmol, 218 mg), to which a solution of CuSO4x5H2O (0.65 mmol, 162 mg) in H2O (1.30 mL) was added. An additional NaHCO3 (109 mg, 1.30 mmol) was added, followed by ditertbutyldicarbonate (1.43 mmol, 312 mg) dissolved in 1.6 mL acetone. The mixture stirred 24 h, then methanol (0.50 mL) was added to the solution, and stirring continued 14 h. To the reaction mixture H2O (1 mL) is added and the product was subsequently filtered, washed with H2O (3×1 mL) and dried to give 286 mg (79% yield) of [(N6-Boc-2,5,5-d3)-lysine]2Cu as pale blue solid. To remove the copper, the pale blue Cu-chelate (286 mg, 0.511 mmol) was suspended in H2O (11 mL). Thereafter, 8-quinolinol (200 mg, 1.38 mmol) was added, and the mixture stirred 20 h (pale salad-green precipitate formation). The suspension was f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com