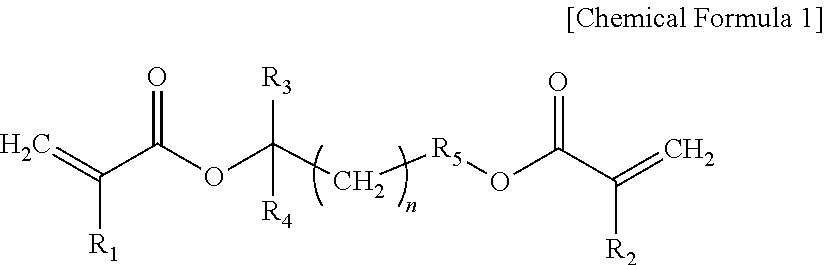
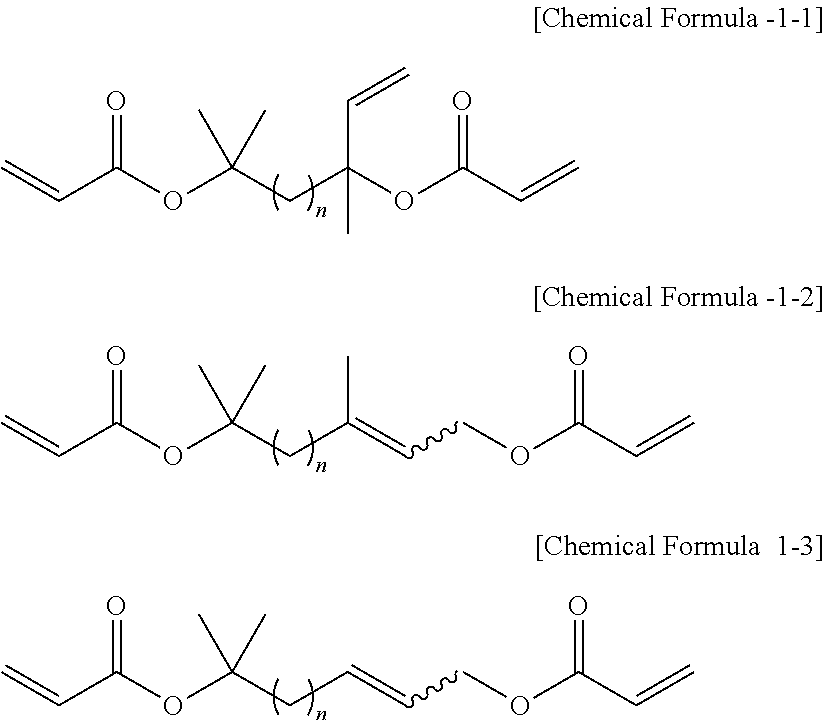
Novel Cross-Linking Agent Compound and Polymer Prepared Using the Same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0075]
synthesis example 1-1
[0076]408.7 g of geraniol was dissolved in 700 ml of ethanol and stirred. Acetic chloride (942 g, 856.4 ml, 4 equiv.) was slowly added dropwise while maintaining the reaction temperature at 30° C. When the conversion and termination of the reaction were confirmed by TLC, the solvent and the unreacted materials were removed by evaporation under reduced pressure. The obtained dichloronate compounds (a mixture of Pc and Tc) were used in the next reaction without any further purification.
[0077]Pc (CDCl3, 500 MHz): 5.50-5.45 (m, 1H), 4.13-4.07 (m, 2H), 2.16-2.06 (m, 2H), 1.83-1.53 (m, 13H)
[0078]Tc (CDCl3, 500 MHz): 6.00 (dd, J=16.87, 11.00, 1H) 5.27 (d, J=16.87, 1H), 5.12 (d, J=11.00, 1H), 2.16-2.06 (m, 2H), 1.83-1.53 (m, 13H).
synthesis example 1-2
[0079]The dichloronate compounds (a mixture of Pc and Tc, 522.9 g, reference material) obtained in Synthesis Example 1-1 were put into an acetone aqueous solution (1.5 L) of about 80% purity, ZnO (427.2 g, 2.1 equiv.) was added, and the mixture was refluxed at a temperature of 100° C. When the conversion and termination of the reaction were confirmed by TLC, the temperature was cooled to room temperature. Thereafter, the solid precipitate was removed using a filtration filter, and the remaining acetone was removed by evaporation under reduced pressure. The remaining organic material and a small amount of water were removed by fractional distillation to obtain desired diol compounds (a mixture of Pa and Ta).
[0080]Pa (CDCl3, 500 MHz): 5.43-5.41 (m, 1H), 4.17-4.12 (m, 2H), 2.12-1.97 (m, 2H), 1.75-1.13 (m, 13H)
[0081]Ta (CDCl3, 500 MHz): 5.91 (dd, J=17.10, 10.52, 1H) 5.22 (d, J=17.09, 1H), 5.07 (d, J=10.52, 1H), 2.06-1.97 (m, 2H), 1.76-1.13 (m, 13H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Chemical formula | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com