Differentiation of lyme disease and southern tick-associated rash illness
a technology of southern ticks and differential markers, applied in the field of human disease detection tools and methods, can solve the problems that i>b. burgdorferi /i>antibodies cannot be used as reliable differential markers for stari, no laboratory tools or methods exist for diagnosis, and poor sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
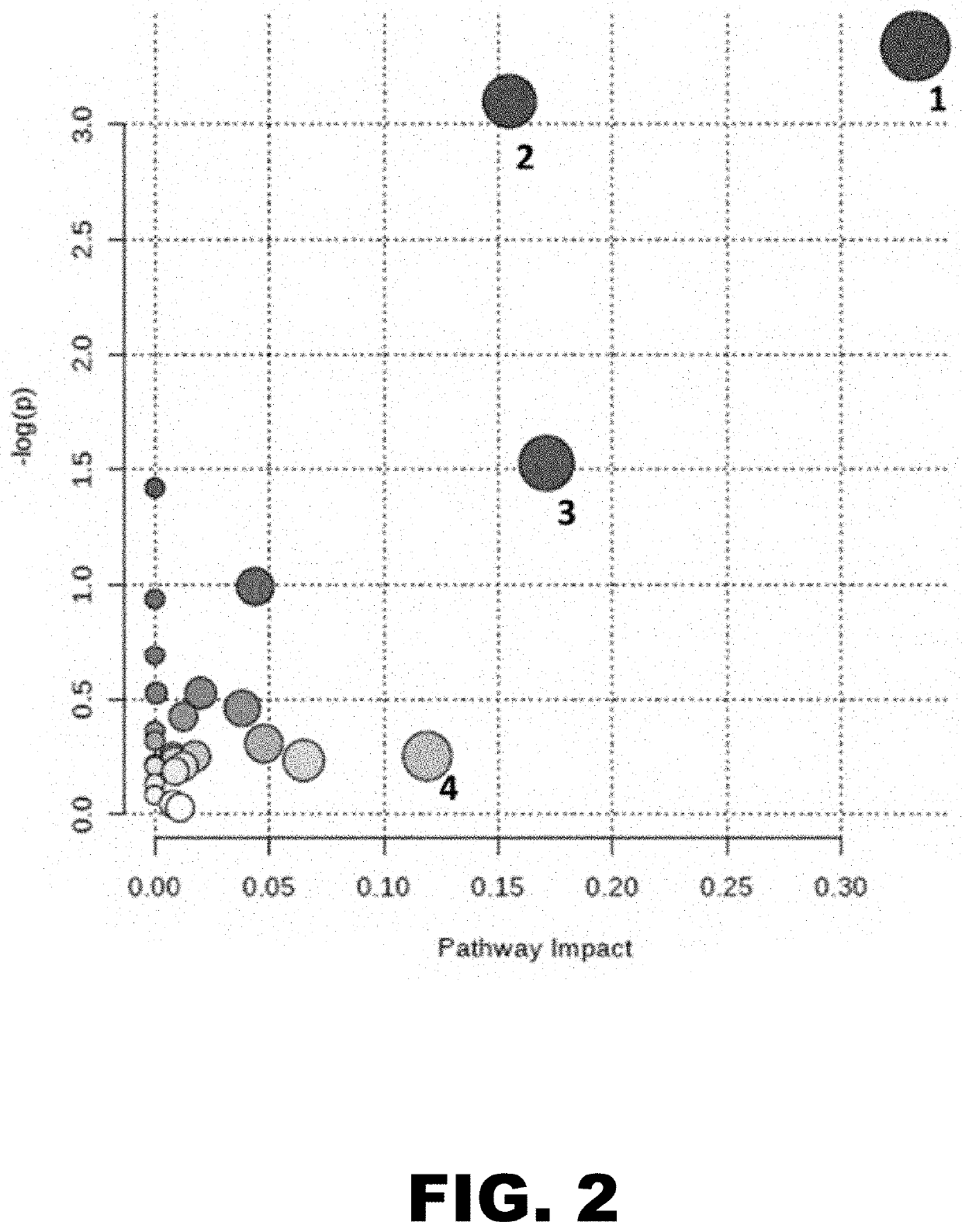
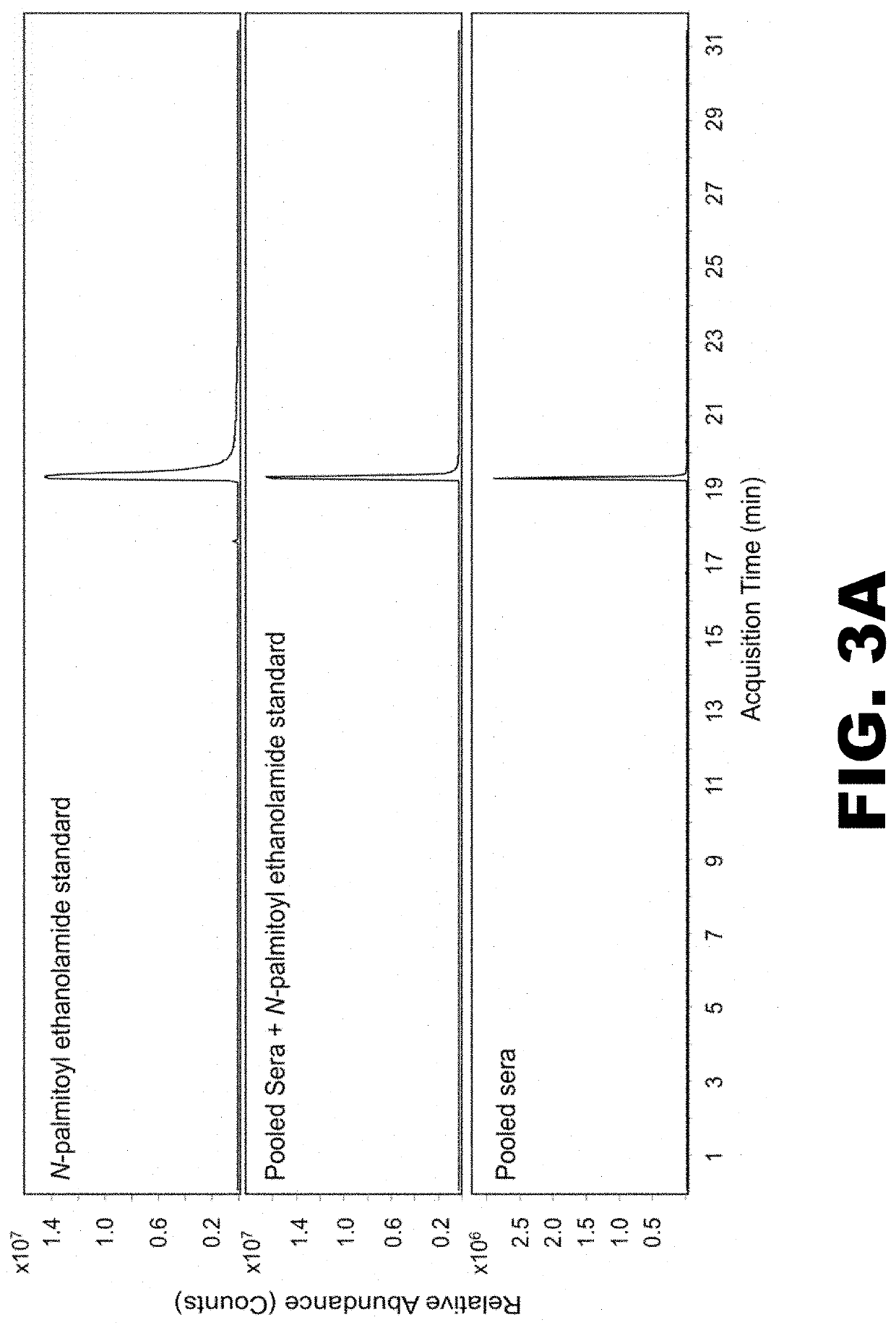
[0085]Lyme disease is a multisystem bacterial infection that in the United States is primarily caused by infection with Borrelia burgdorferi sensu stricto. Over 300,000 cases of Lyme disease are estimated to occur annually in the United States, with over 3.4 million laboratory diagnostic tests performed each year (1, 2). Symptoms associated with this infection include fever, chills, headache, fatigue, muscle and joint aches, and swollen lymph nodes; however, the most prominent clinical manifestation in the early stage is the presence of one or more erythema migrans (EM) skin lesions (3). This annular, expanding erythematous skin lesion occurs at the site of the tick bite in 70 to 80% of infected individuals and is typically 5 cm or more in diameter (4, 5). Although an EM lesion is a hallmark for Lyme disease, other types of skin lesions can be confused with EM (3, 5, 6). These include rashes caused by tick-bite hypersensitivity reactions, certain cutaneous fungal infections, bacteri...
example 2
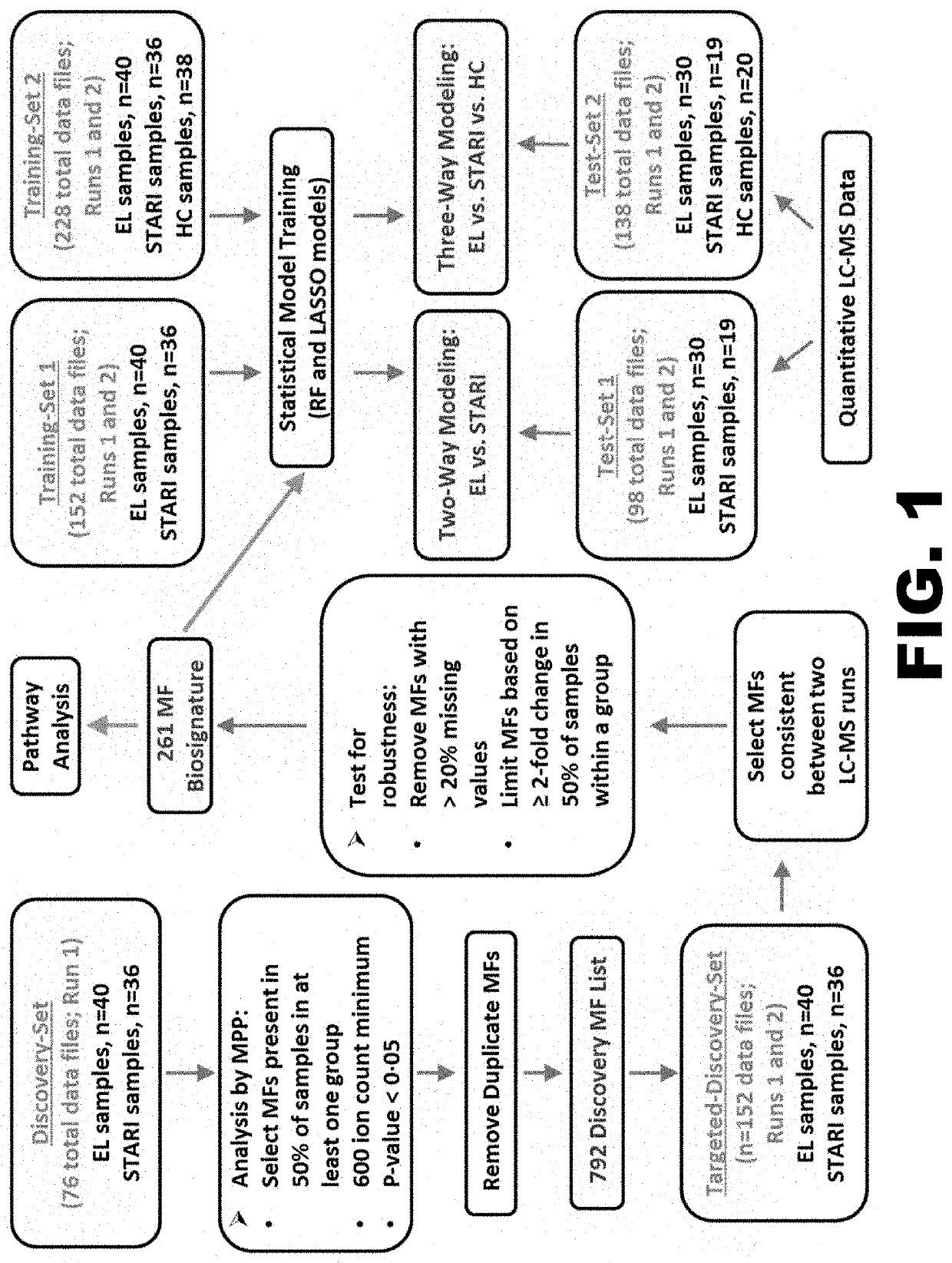
[0108]STARI is an illness that has received little attention over the years, but is a confounding factor in diagnosing early Lyme disease in areas where both illnesses overlap and contributes to the debate surrounding the presence of Lyme disease in the southern United States. No diagnostic tool exists for STARI or for differentiating early Lyme disease from STARI. Based on documented differences between early Lyme disease and STARI (9, 16, 56), we metabolically profiled serum to develop a biochemical biosignature that when applied could accurately classify early Lyme disease and STARI patients (See Example 1). This example describes the design of the study described in Example 1.
[0109]An unbiased-metabolomics study was designed to directly compare the metabolic host responses between these two illnesses, and subsequently evaluate how this metabolic biosignature distinguishes these two illnesses. The use of unbiased metabolomics for biosignature discovery does not lend itself to pow...
example 3
[0113]This example describes methods used for Lyme disease serologic testing of all serum samples used in the examples above. Standard two-tiered testing was performed on all samples (38). The C6 B. burgdorferi (Lyme) ELISA (Immunetics, Boston, Mass.) was used as a first-tier test, and any positive or equivocal samples were reflexed to Marblot IgM and IgG immunoblots (MarDx Diagnostics, Inc., Carlsbad, Calif.) as the second-tier test. Serologic assays were performed according to the manufacturer's instructions, and the data were interpreted according to established CDC guidelines (38). Duration of illness, however, was not considered for test interpretation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com