New dosage form of selumetinib and derivative of selumetinib and preparation method of new dosage form
A technology of selumetinib and its derivatives, which is applied in the field of pharmaceutical preparations, can solve problems such as limited application and leukopenia, and achieve the effects of reducing drug dosage, fast onset of action, and reducing drug burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
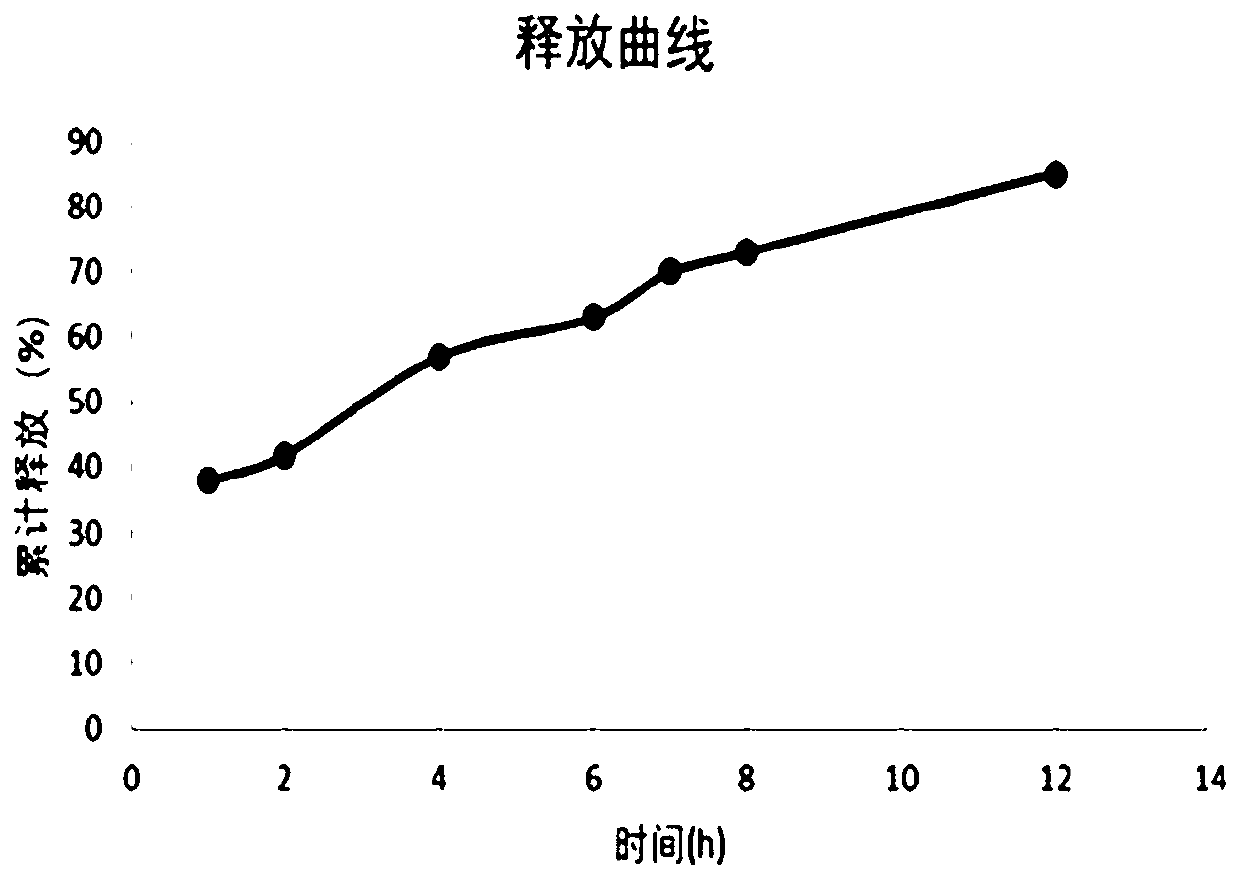
Embodiment 1
[0067] Preparation of tablet of the present invention (selumetinib molar ratio 70:30 in the constant release part and the sustained release part)
[0068] Raw materials: 20g of selumetinib hydrochloride, 64.8g of hydroxypropyl methylcellulose (HPMC K4M), 110g of lactose, 100g of starch, 5g of microcrystalline cellulose (1000 pieces), the preparation method is as follows:
[0069] a, get selumetinib hydrochloride 6g, add hydroxypropyl methylcellulose (HPMC K4M) and lactose, pulverize, pass through a 100-mesh sieve after being ground into fine powder;
[0070] b. Add appropriate amount of ethanol to make a film;
[0071] c. Dry in a dryer at 85°C for 20 minutes;
[0072] d. Tablet pressing with a tablet press to prepare the inner core of the slow-release part;
[0073] e, get selumetinib hydrochloride 14g, add microcrystalline cellulose to pulverize, pass 100 mesh sieves after being ground into fine powder;
[0074] f. Add appropriate amount of sterile water to make a soft ta...
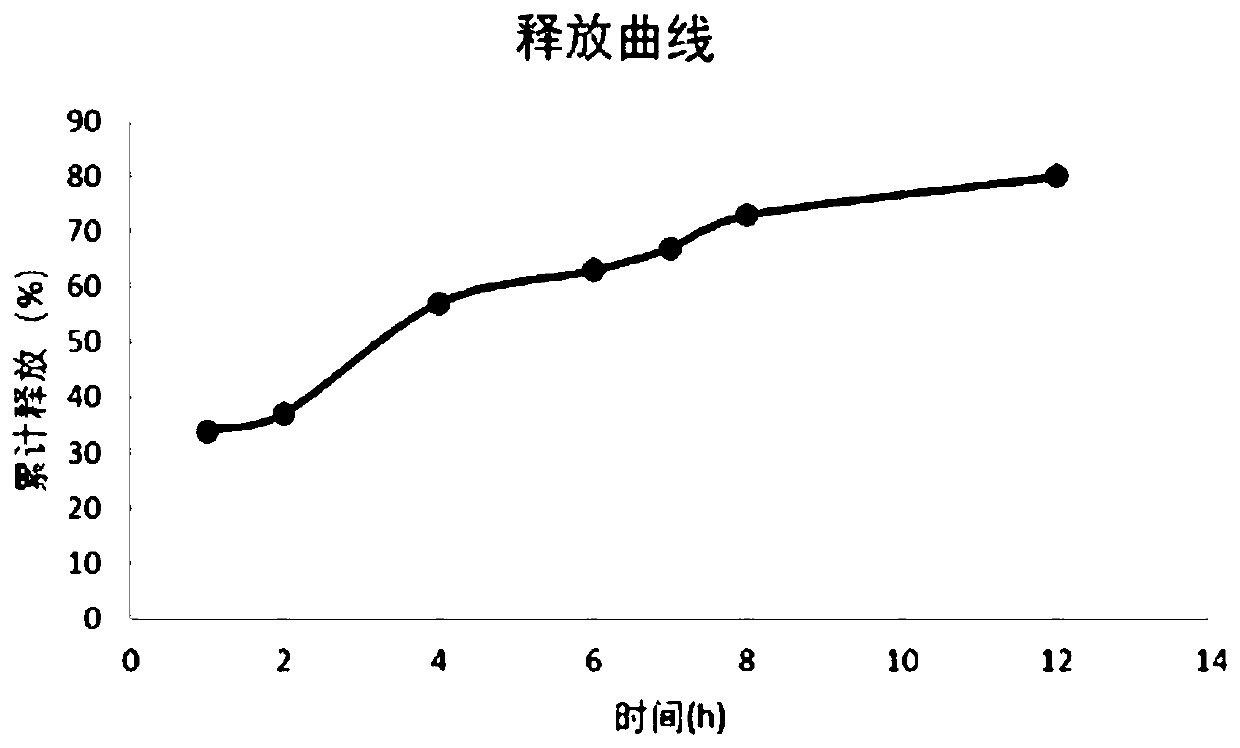
Embodiment 2
[0078] Preparation of tablet of the present invention (selumetinib molar ratio 65:35 in the constant release part and the sustained release part)
[0079] Raw materials: 40g of selumetinib hydrochloride, 20g of tragacanth gum, 1.5g of flavoring agent, appropriate amount of distilled water, 80g of starch, 20g of dextrin (1000 tablets), the preparation method is as follows:
[0080] a. Take 14 g of selumetinib hydrochloride, add tragacanth gum, pulverize, grind into fine powder and pass through a 100-mesh sieve;
[0081] b. Add appropriate amount of ethanol to make a film;
[0082] c. Dry in a dryer at 85°C for 20 minutes;
[0083] d. Tablet pressing with a tablet press to prepare the inner core of the slow-release part;
[0084] e, get selumetinib hydrochloride 26g, add dextrin, grind into a fine powder and pass through a 100-mesh sieve;
[0085] f. Add appropriate amount of sterile water to make a soft tablet, and coat the outer layer of the tablet core obtained in step d t...
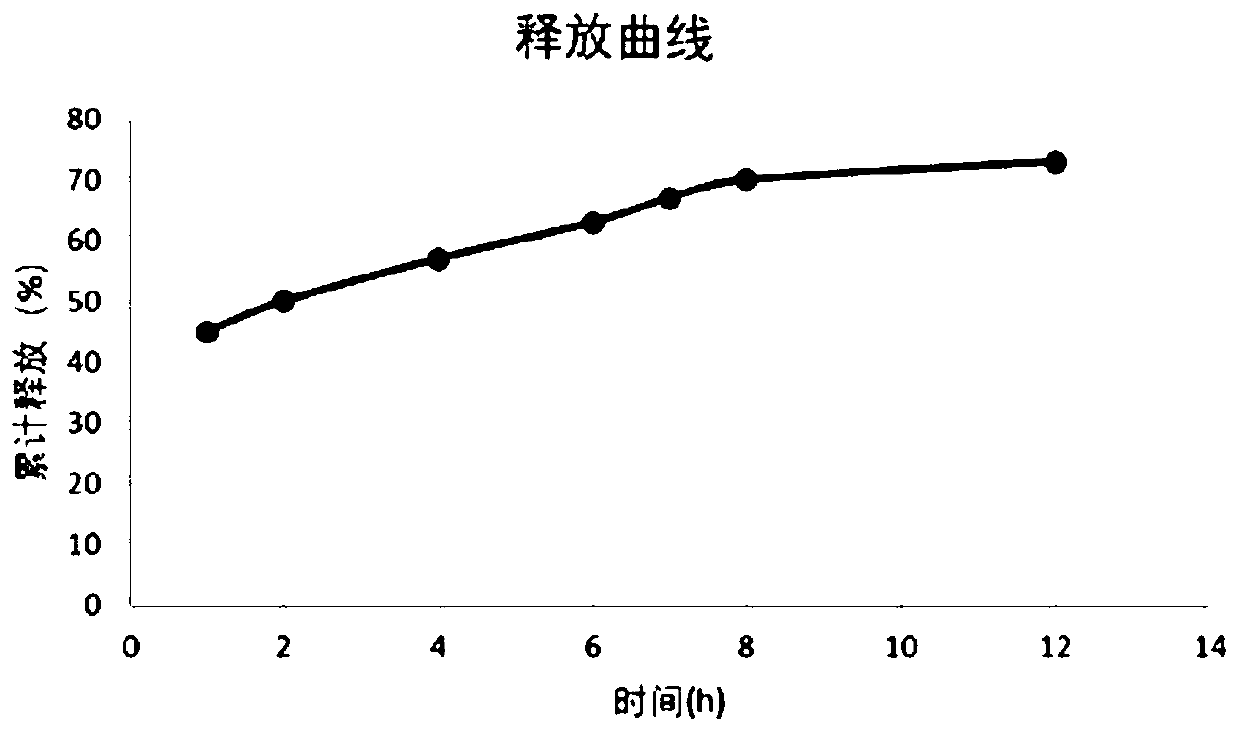
Embodiment 3
[0089] Preparation of tablet of the present invention (selumetinib molar ratio 75:25 in the constant release part and the sustained release part)
[0090] Raw materials: 40g of selumetinib hydrochloride, 50g of hydroxypropyl methylcellulose (HPMC K100M), 45g of methylcellulose, 80g of starch, 20g of microcrystalline cellulose (1000 pieces), the preparation method is as follows:
[0091] a, get selumetinib hydrochloride 10g, add hydroxypropyl methylcellulose (HPMC K4M) and methylcellulose and starch, pulverize, cross 100 mesh sieves after being ground into fine powder;
[0092] b. Add appropriate amount of ethanol to make a film;
[0093] c. Dry in a dryer at 85°C for 20 minutes;
[0094] d. Tablet pressing with a tablet press to prepare the inner core of the slow-release part;
[0095] e, get 30g of selumetinib hydrochloride, add microcrystalline cellulose to pulverize, pass through a 100-mesh sieve after being ground into fine powder;
[0096] f. Add appropriate amount of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com