Lipid nanoparticles as oral vehicles of immunotherapy
a technology of lipid nanoparticles and immunotherapy, which is applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of t1d autoreactivity development that is much longer, remains quite elusive, and is not easy to be detected and treated, and achieves the potential for local and systemic pathophysiologic effects, low bioavailability, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0111]Targeted Jak inhibition synergizes with CoB to prolong transplant survival.
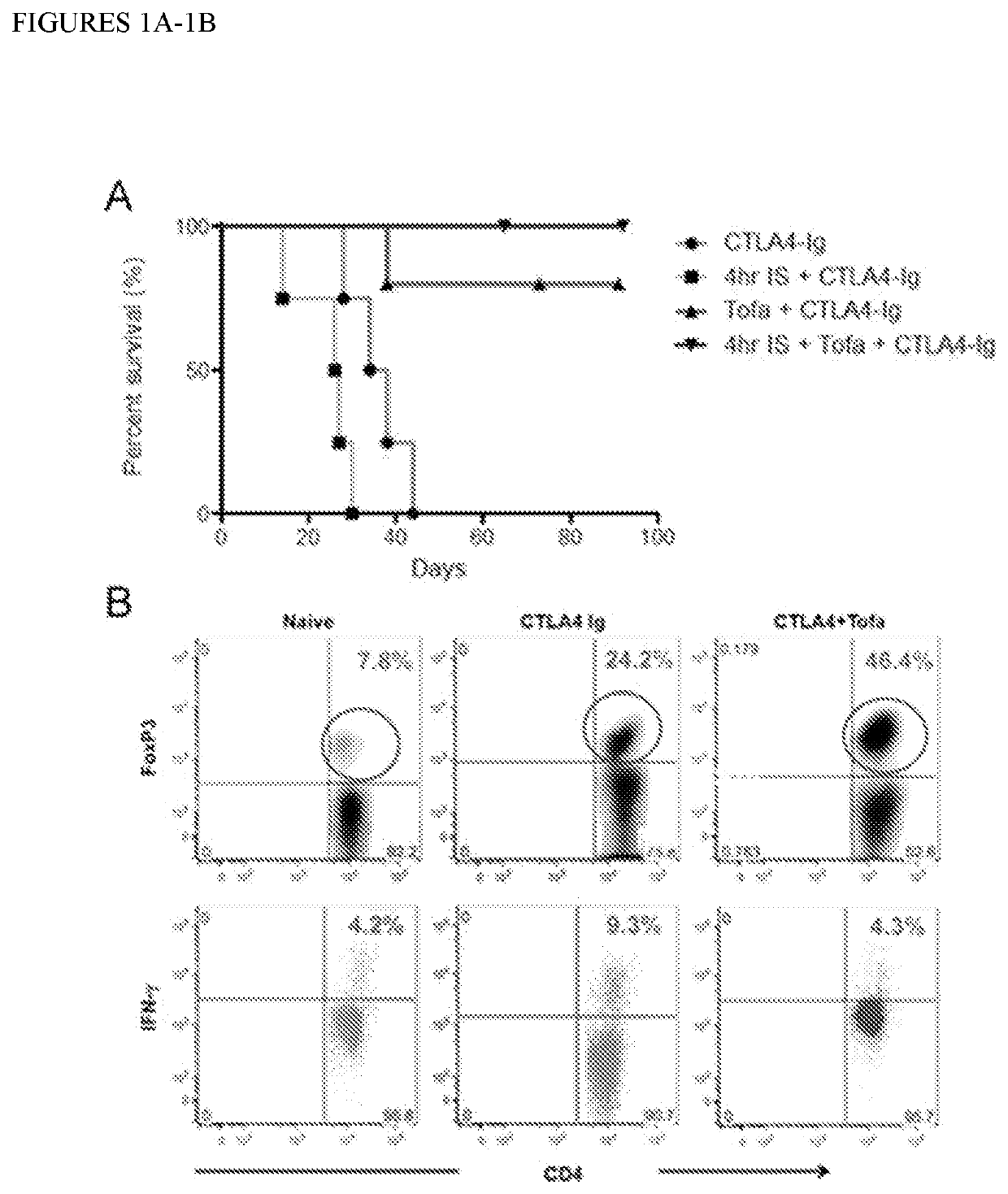
[0112]The feasibility of combining CTLA4-Ig with a short-course Tofa administration in a full MHC mismatch (C57BL / 6 to BALB / c) mouse heart transplantation model was tested (FIG. 1A). Encouragingly, heart transplant survival in mice treated with CTLA-4Ig was significantly improved by a short course (10 days) of Tofa (MST untreated: 9d, CTLA4-Ig only: 36d, Tofa+CTLA4-Ig: 120d). This improvement was evident even in mice receiving hearts kept ischemic for 4 h before transplantation—a more clinically relevant scenario—where CTLA4-Ig efficacy is undermined by early accumulation of inflammatory mediators67 (MST untreated: 9d, CTLA4-Ig only: 26.5d, Tofa+CTLA4-Ig: 150d). Analysis of the graft infiltrate by flow cytometry revealed that the protective effect of the combination of Tofa+CTLA4-Ig was associated the accumulation of a much higher frequency of Treg cells (FIG. 1B). Moreover, ex-vivo restimulation of spl...
example 2
[0113]Targeted Jak inhibition limits DC maturation.
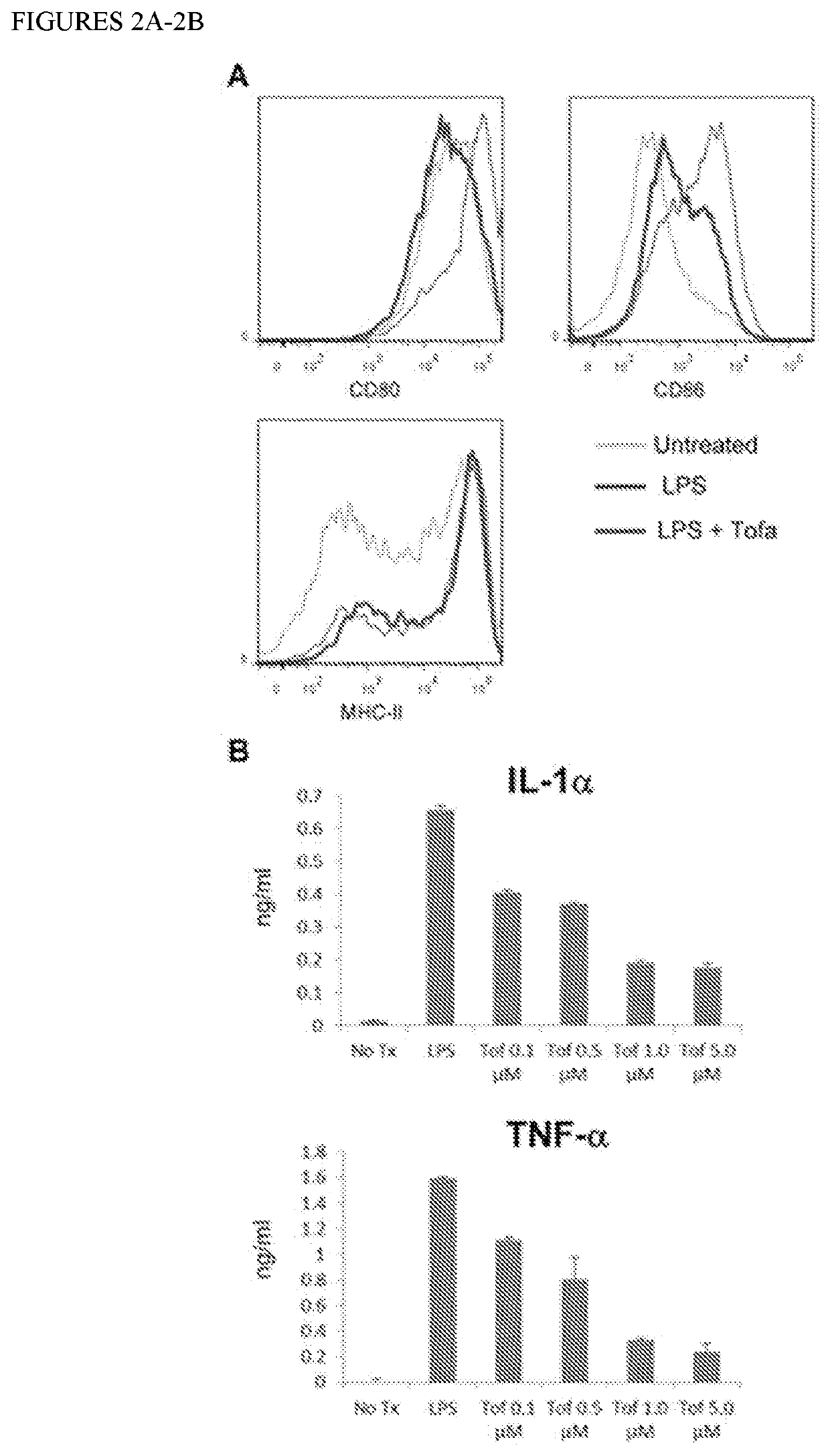
[0114]To better understand the impressive protective effect achieved with the combination Tofa+CTLA4-Ig, we tested the ability of Tofa to inhibit the maturation of bone marrow derived dendritic cells (DC) in response to LPS (FIG. 2). Exposure to Tofa caused a significant inhibition of the up-regulation of CD80 and CD86 costimulatory molecules, with a concomitant reduction in release of the prototypical inflammatory cytokines IL-6 (not shown), IL-1, and TNF-α. The latter two have been implicated in T1D development and inhibition of their accumulation would be a valuable effect. Interestingly, Tofa did not prevent the up-regulation of MHC-II molecules, rendering DC with high Signal 1 but very low Signal 2 (costimulatory molecules) and low Signal 3 (cytokines)—a phenotype that could maximize the impact of CTLA4-Ig on reactive T cells.
example 3
[0115]Tofa does not interfere with Treg suppression of T cells.
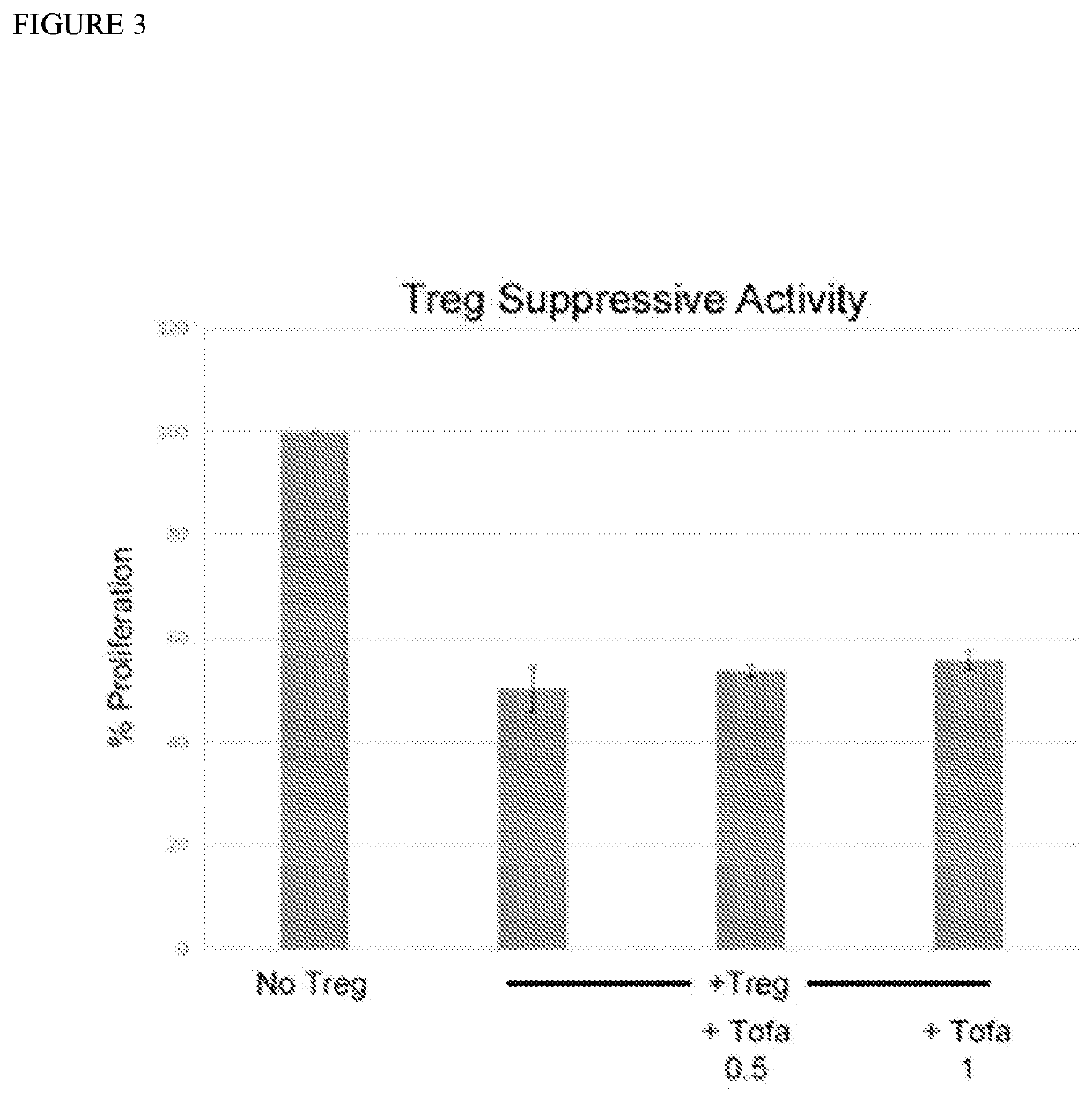
[0116]The observed accumulation of Treg in transplants treated with Tofa+CTLA4-Ig warranted confirmation that Tofa (although transient) would not interfere with Treg function. The results of a T cell CFSE-Proliferation-Assay conducted to assess the suppressive activity of Treg (added at either 2:1 or 4:1 T cell to Treg ratio) without and with Tofa (at 0.5 or 1 μM) confirmed the preservation of regulatory activity (FIG. 3). These results show the potential of the combined use of Tofa with CTLA4-Ig for induction of long-term regulation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com