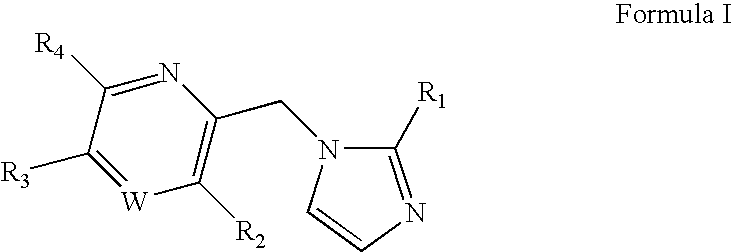
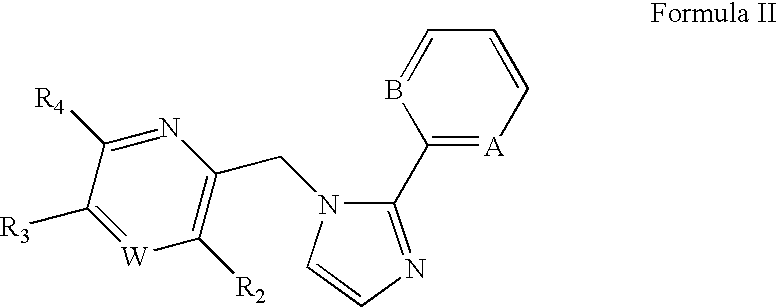
Substituted imidazolylmethyl pyridine and pyrazine derivatives GABAA receptor ligands
a technology of imidazolylmethyl pyridine and pyrazine derivatives, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of compound known to exhibit a number of unwanted side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Representative Imidazolylmethyl Pyridines
[0126]This Example illustrates the synthesis of 2-[2-(6-fluoro-pyridin-2-yl)-imidazol-1-yl methyl]-3,5-diethoxy-pyridine (compound 4), a representative imidazolylmethyl pyridine.
1. Preparation of 3,5-Diethoxy-pyridine-2-carboxylic acid ethyl ester
[0127]3,5-dibromo-pyridine-2-carboxylic acid methyl ester is prepared using published protocols (Bull. Chem. Soc. Japan (1970) 43:3210–3214), as summarized above. To a stirred solution of Na (78 mg, 3.39 mmol) in EtOH (5 mL), 3,5-dibromo-pyridine-2-carboxylic acid methyl ester (0.5 g, 1.69 mmol) is added, and refluxed for 5 hours. After evaporation of most of the solvent under reduced pressure, the residue is neutralized with saturated NH4Cl solution, extracted with EtOAc, washed with brine, dried over Na2SO4, and concentrated. The organic residue is purified with 50% EtOAc / hexanes to afford 3,5-diethoxy-pyridine-2-carboxylic acid ethyl ester as a viscous liquid (250 mg, 60%); 1H NMR ...
example 2
Preparation of Representative Imidazolylmethyl Pyrazines
[0131]This Example illustrates the synthesis of representative imidazolylmethyl pyrazines.
A. 2-[2-(6-Fluoro-pyridin-2-yl)-imidazol-1-ylmethyl]-3-methoxy-5-phenyl-pyrazine (Compound 1)
1. Preparation of 3-Methoxy-5-phenyl-pyrazine-2-carboxylic acid methyl ester
[0132]To a solution of 2-amino-malononitrile 4-toluenesufonate (5.0 g, 19.3 mmol) in methanol (60 mL) at room temperature under N2 is added NaOMe (25% w / w in MeOH, 5.2 mL, 22.7 mmol). After 2 hours, acetic acid (0.204 g, 3.4 mmol) is added. The mixture is cooled to 0° C. and to the mixture is added benzaldehyde (3 g, 19.9 mmol). The mixture is stirred at this temperature for an additional 2 hours and then gradually warmed to room temperature overnight. To the mixture is added concentrated HCl (9.2 g, 80 mmol) and the mixture is stirred at room temperature over the weekend. Solvent is removed in vacuo. To the crude mixture is added saturated NH4Cl (50 mL) and DCM (100 mL). ...
example 3
Ligand Binding Assay
[0163]The high affinity of compounds of this invention for the benzodiazepine site of the GABAA receptor was confirmed using a binding assay essentially described by Thomas and Tallman (J. Bio. Chem. (1981) 156:9838–9842, and J. Neurosci. (1983) 3:433–440).
[0164]Rat cortical tissue was dissected and homogenized in 25 volumes (w / v) of Buffer A (0.05 M Tris HCl buffer, pH 7.4 at 4° C.). The tissue homogenate was centrifuged in the cold (4° C.) at 20,000×g for 20 minutes. The supernatant was decanted, the pellet rehomogenized in the same volume of buffer, and centrifuged again at 20,000×g. The supernatant of this centrifugation step was decanted and the pellet stored at −20° C. overnight. The pellet was then thawed and resuspended in 25 volumes of Buffer A (original wt / vol), centrifuged at 20,000×g and the supernatant decanted. This wash step was repeated once. The pellet was finally resuspended in 50 volumes of Buffer A.
[0165]Incubations contained 100 μl of tissue ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com