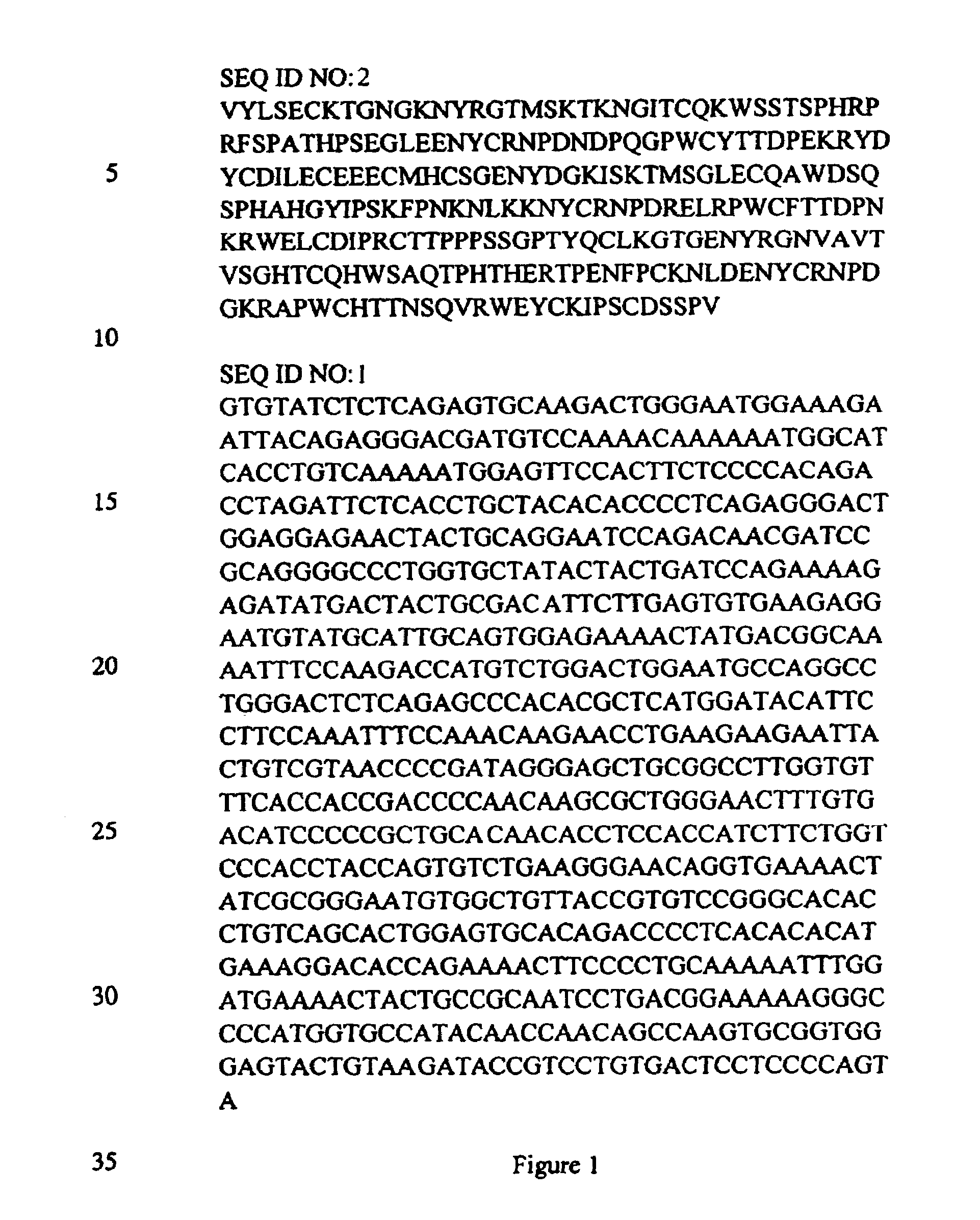
Deglycosylated kringle 1-3 region fragments of plasminogen and methods of use
a plasminogen and region fragment technology, applied in the field of deglycosylated kringle 13 region proteins, can solve the problems of abnormal or undesired angiogenesis, and achieve the effects of modulating angiogenesis, increasing the anti-angiogenic activity of these proteins, and inhibiting unwanted angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Glycosylated and Deglycosylated Kringle 1-3 Region Proteins
[0091]Human plasminogen was purified using affinity chromatography as described in Brockway, W. J. and Castellino, F. J., “Measurement and the binding of antifibrinolytic amino acids to various plasminogens”Arch. Biochem. Biophys. 151:194–199 (1972). One liter of human plasma (Children's Hospital blood bank) was applied to a 200 mL lys-Sepharose 4b (Pharmacia) column at a flow rate of 2–3 ml / minute. The column, previously equilibrated with 50 mM Tris pH 7.4 (Sigma) was then washed with 500 mL 50 mM tris / 1M NaCl pH 7.4. Bound plasminogen was eluted with 200 mL 50 m M tris / 200 mM ε-aminocaproic acid (εACA). The purity of this material was greater than 95% as assessed by SDS-PAGE. Human plasminogen was further fractionated into glycosylated plasminogen (plasminogen 1) and deglycosylated plasminogen (plasminogen 2) using lectin affinity chromatography. Human plasminogen (10 mg) was applied to a 5 mL conA HiTrap colu...
example 2
Deglycosylation of Plasminogen Results in an Increased Yield of Kringle 1-3 Region Proteins
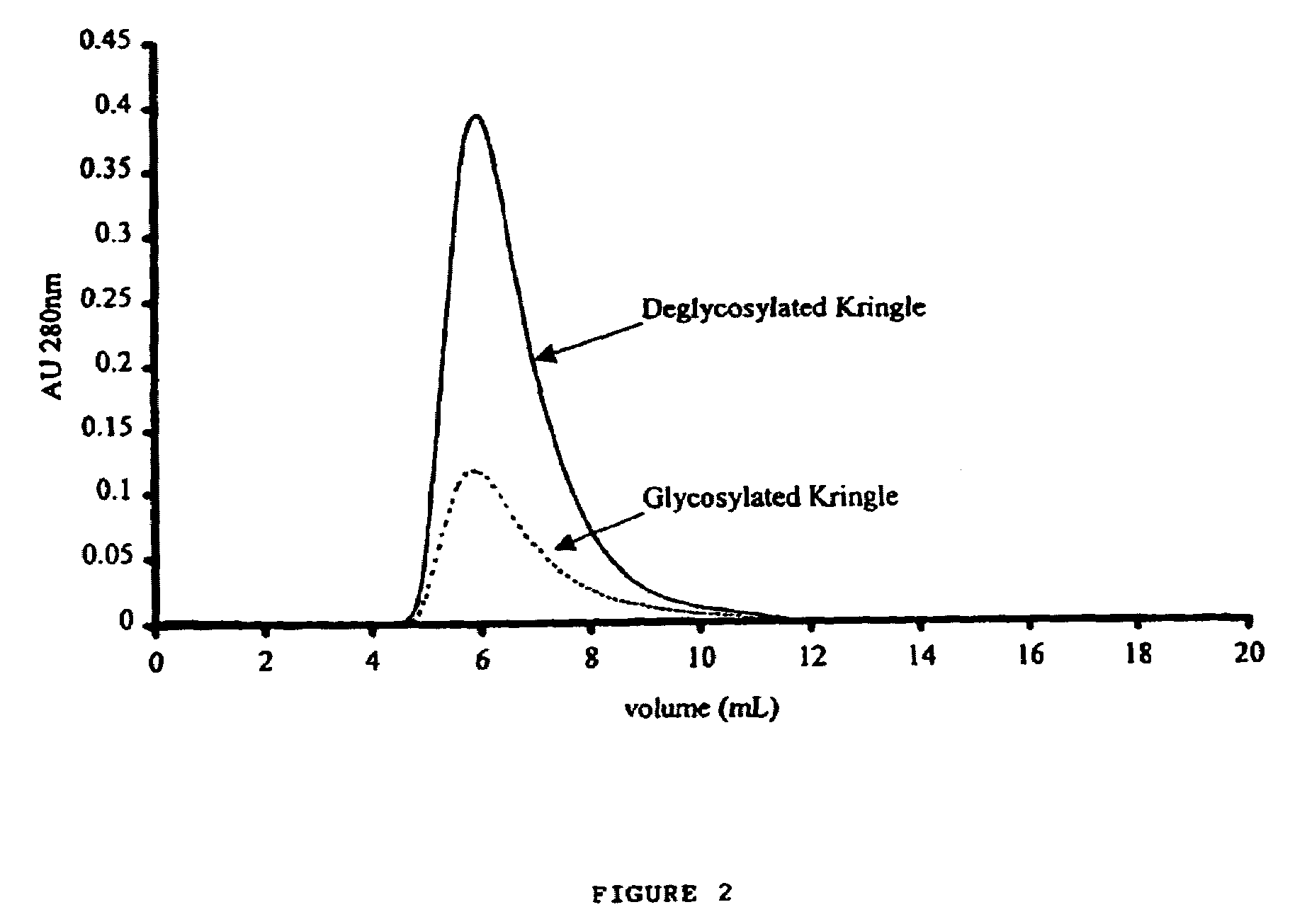
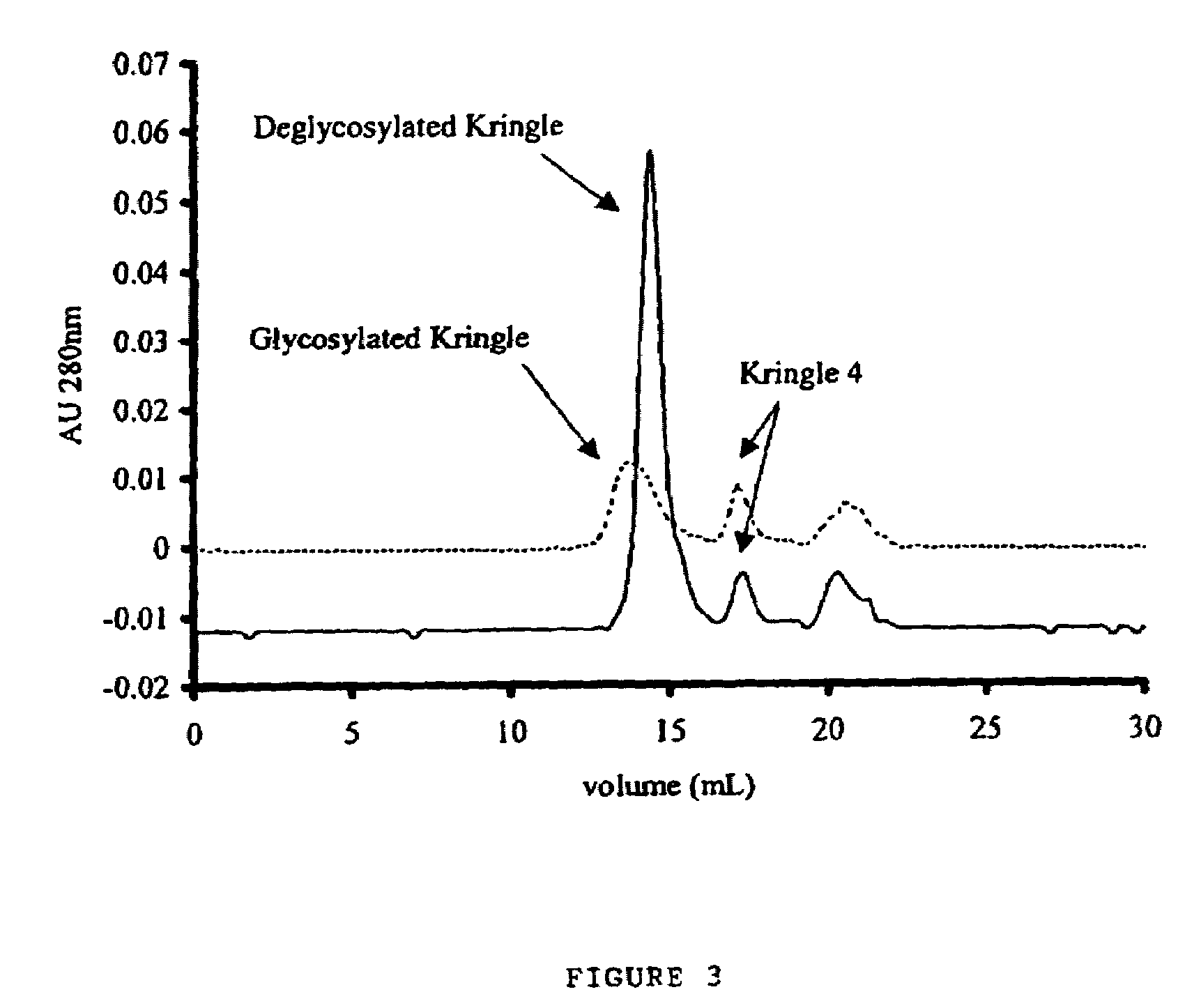
[0094]Equal amounts of plasminogen 1 and plasminogen 2 were digested with PPE, and the kringle 1-3 region proteins purified as described in Example 1 using a combination of affinity chromatography (lys-Sepharose) to purify kringle 1-3 region proteins and other lysine binding fragments of plasminogen, and gel filtration (Superdex200) to separate kringle 1-3 region proteins from kringle 4. FIG. 2 shows a typical chromatogram from the affinity column, demonstrating the reduced yield obtained when plasminogen 1 is used, as a substrate compared to plasminogen 2. FIG. 3 shows the chromatogram obtained from a typical gel filtration experiment as described in Example 1). It was noted that kringle 1-3 region protein from plasminogen I has a slightly lower retention time (13.77 minutes) than deglycosylated kringle 1-3 region protein (14.36 minutes), as would be expected from this glycosylated fragment. ...
example 3
Deglycosylated Kringle 1-3 Region Proteins Have Increased Antiangiogenic Activity as Compared to Glycosylated Kringle 1-3 Region Proteins
[0097]Both kringle 1-3 region protein glycoforms were assayed for their ability to inhibit the proliferation of bovine endothelial cells as described in Example 1. Both kringle 1-3 region protein glycoforms inhibited endothelial cell proliferation. However, glycosylated kringle 1-3 region protein, which contains an N-linked carbohydrate, has a measured IC50 value of approximately 13 μg / mL, whereas deglycosylated kringle 1-3 region protein, the glycoform, lacking an N-linked carbohydrate, has a measured IC50 value of approximately 2.4 μg / nL. Thus, deglycosylated kringle 1-3 region protein appears to be a much more efficient inhibitor of endothelial cell proliferation, and in particular was 4–5 fold more efficient as an inhibitor of endothelial cell proliferation.
[0098]All patents, publications and abstracts cited above are hereby incorporated by ref...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com