59R mutant vector for expressing rFC protein as well as preparation method and application of 59R mutant vector
A protein and vector technology, applied in the field of 59R mutant vector and its preparation, can solve the problems such as difficulty in obtaining soluble expression products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The present embodiment is the carrier construction of horseshoe crab C protein gene (rFC):
[0031] (1) Get the N-terminal sequence of the Nephila pilipes protein with better fiber properties on GeneBank, and use it as the wild-type sNT (gene sequence); carry out the 59th amino acid sequence of the wild-type sNT Mutated to obtain the sNTD59R sequence, the amino acid sequence of the mutated sequence is shown in the sequence table SEQ: NO: 1:
[0032] (2) Extract Limulus Chinese Limulus blood, clone Limulus Limulus C protein gene (rFC) and sequence, obtain Limulus Limulus C protein gene (rFC), its sequence is as shown in the sequence table SEQ: NO: 2:
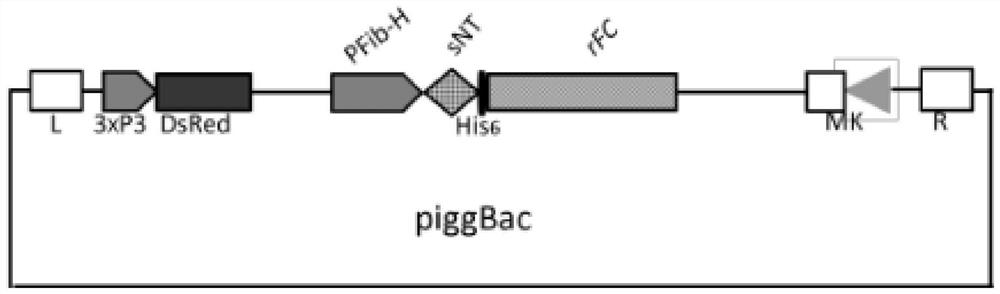
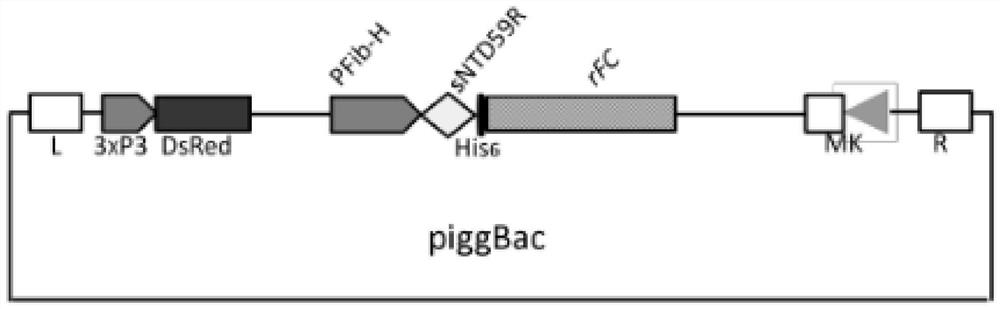
[0033] Construct two vectors according to the above steps, and their ligation sequence is as follows: figure 1 with figure 2 Shown:
[0034] in, figure 1 It is a schematic diagram of the construction of the wild-type vector (sNT+rFC): in the figure, the transposon of the piggyBac expression vector is connected with th...
Embodiment 2
[0037] This embodiment is the method for transferring the carrier of embodiment 1 into the silk gland:
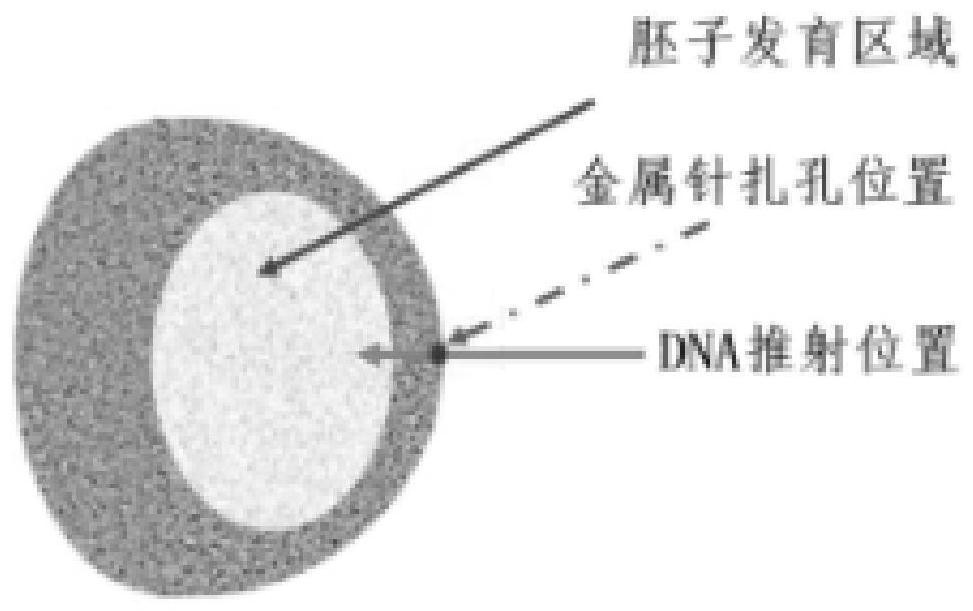
[0038] (1) The two vectors of Example 1 are all injected into the embryo development zone of silkworm eggs by microinjection;
[0039] (2) After the silkworm eggs in step (1) are hatched and raised as worms, the moths with pure red-eye phenotype are screened for 3 consecutive generations, and the third-generation silkworms that do not segregate are selected for feeding, and the moths with pure red-eye gene are bred. transgenic silkworms that are homozygous or rFC protein homozygous and whose silk gland cells can secrete rFC protein;
[0040] (3) The rFC protein was secreted into the silk gland lumen of the silkworm under the action of the signal peptide of the heavy chain of silk fibroin, and then secreted into the cocoon.
[0041] In order to improve the hatching rate, you need to pay attention to the following points when injecting: ① Within the appropriate time range fo...
Embodiment 3
[0043] The rFC protein content is identified in the transgenic silk gland of embodiment 2, specifically as follows:
[0044] The silk gland of the 5th instar larvae of the transgenic silkworm was dissected, and the rFC protein was identified by Western Blot (WB); the results were as follows Figure 4 As shown in the figure, it can be seen that the binding fragment of rFC protein has appeared in the western blot, which proves that there is rFC protein in the silk gland. In the figure, the band of wild type (WT) is obviously thinner than that of 59R mutant type (MUT), This proves that the rFC protein expression level of the 59R mutant is much higher than that of the wild type in this experiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com