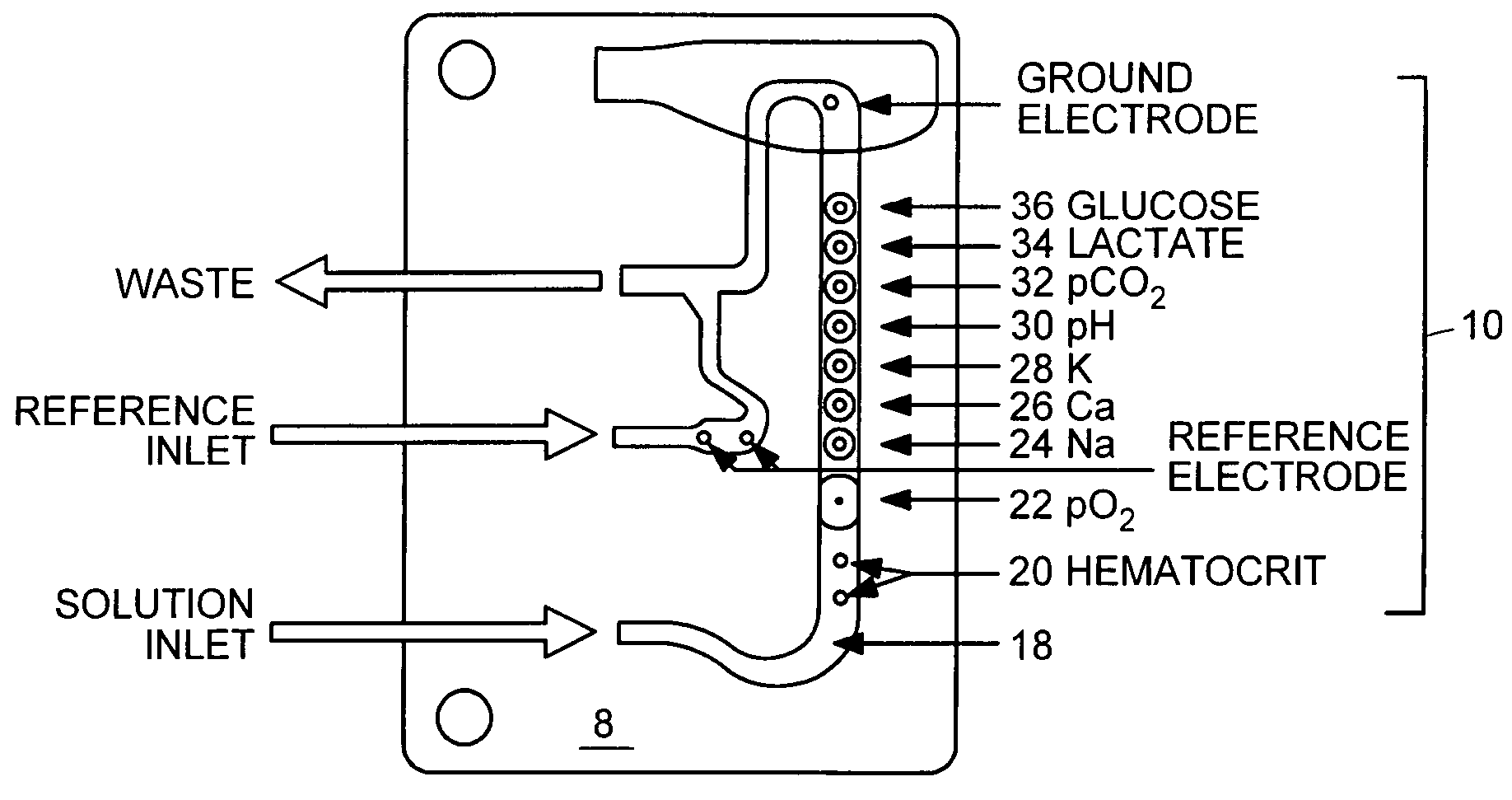
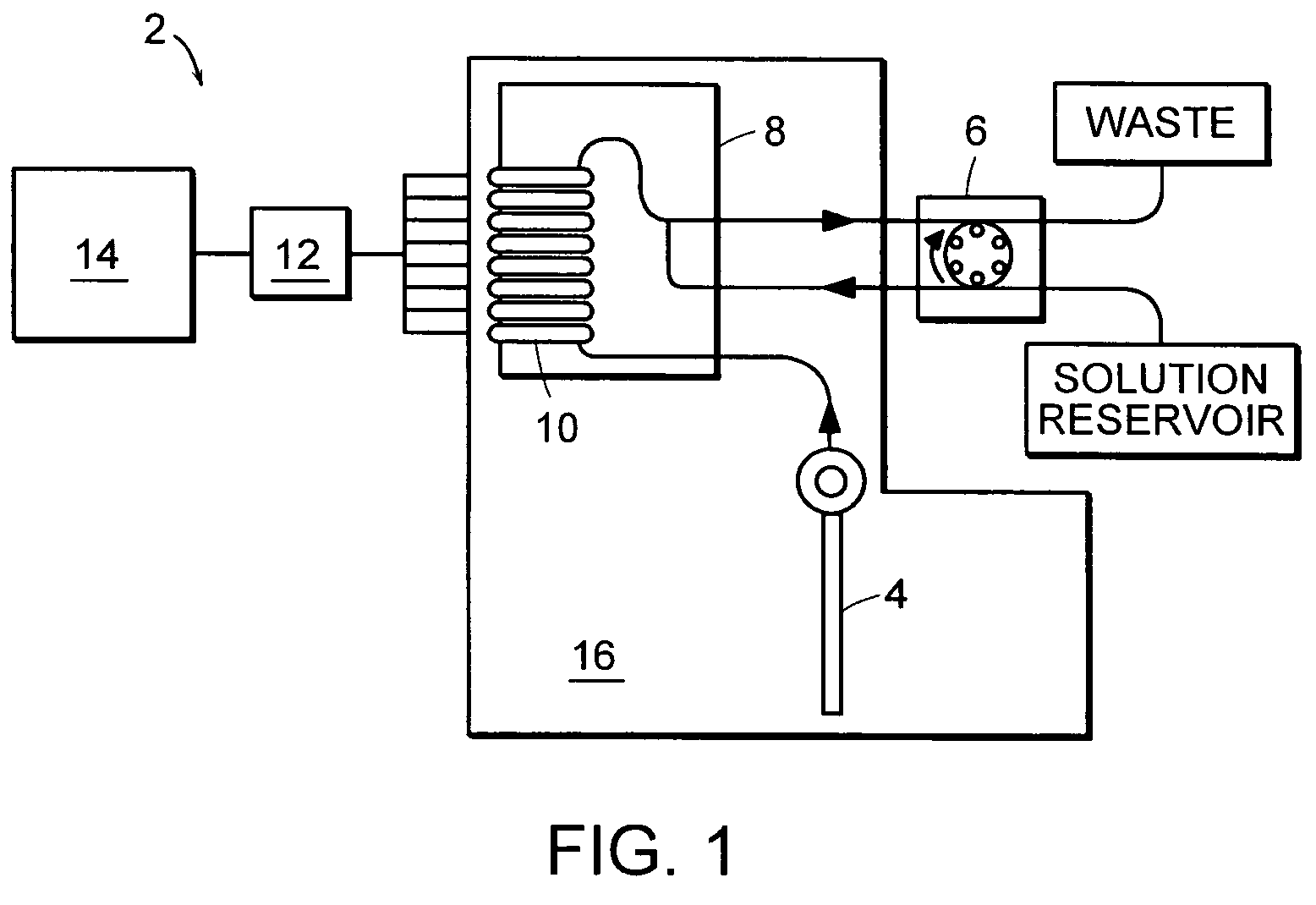
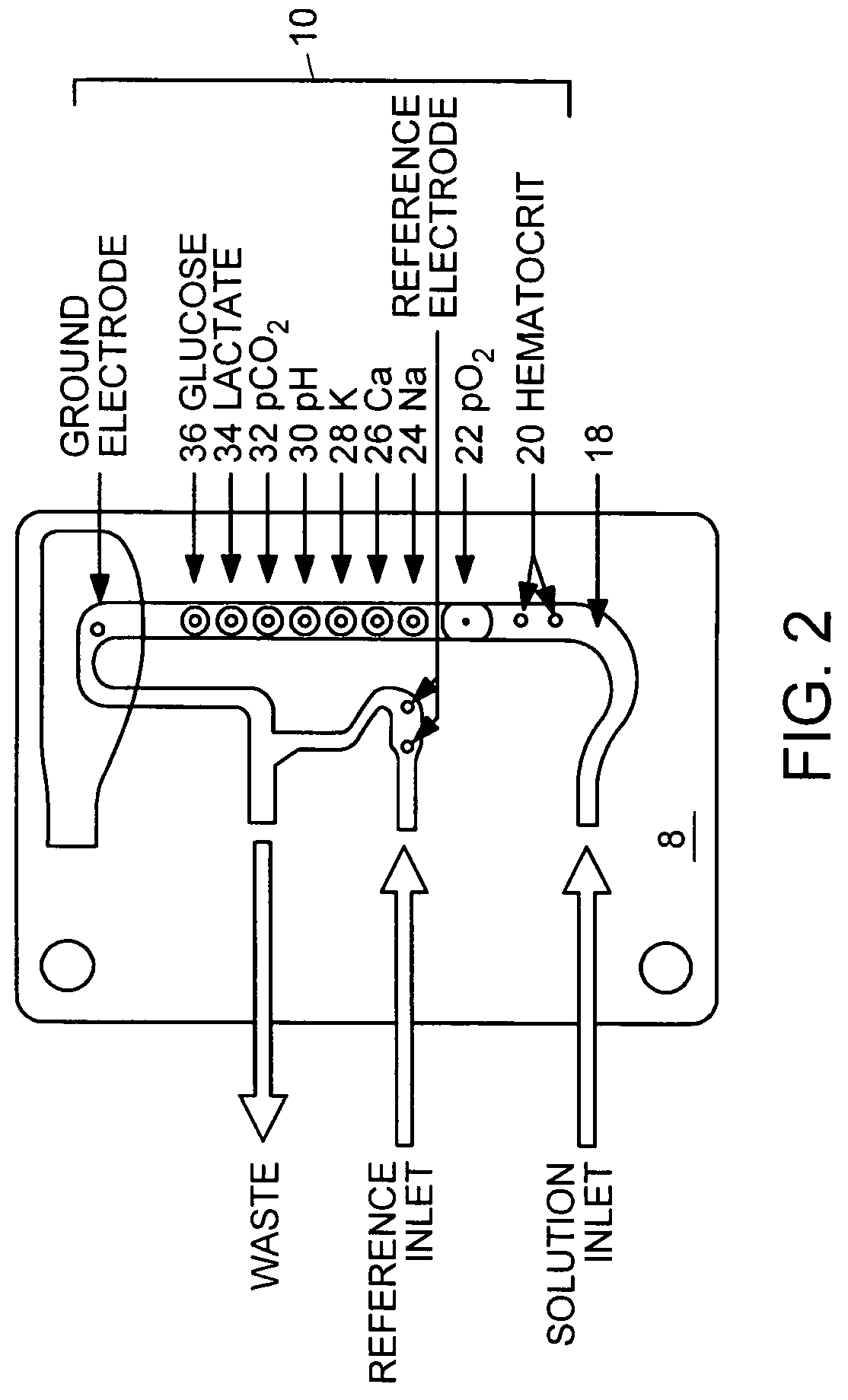
Multi-analyte reference solutions
a reference solution and multi-analyte technology, applied in the field of reference solutions for instruments, can solve the problems of difficult to formulate aqueous solutions with physiological, difficult to maintain tolerable interference levels, and relatively unstable blood products, etc., to achieve the effect of not being highly viscous and maintaining tolerable interference levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045]A reference solution was formulated according to Table 1 below:
[0046]
TABLE 1Deionized Water1.00 LHEPES buffer 100 mmolNaOH 75 mmolNaCl 80 mmolKCl 7.0 mmolCaCl21.00 mmolChloline Chloride 1 mmolGlucose 12 mmolLithium Lactate 4 mmolMIT biocide 2.0 mmolFD&C Blue No. 10.01 gFD&C Yellow No. 50.03 gPEG (MW 2000) 90 gDextran (MW 10,000) 60 gEthylene Glycol 90 g
[0047]The reference solution was introduced to a blood analyzer containing an electrode card equipped with sensors to detect pH, carbon dioxide (CO2), oxygen (O2), sodium (Na), potassium (K), calcium (Ca), glucose (Glu), lactate (Lac), and hematocrit (Hct). Three hematocrit values were obtained, along with three concentration values for each analyte. After the final measurement, the electrode card was replaced and the procedure was repeated with a new electrode card containing the same type of electrodes. In some instances, two measurements were recorded with each electrode card, and in others one measurement was recorde...
example 2
[0049]A reference solution was formulated according to Table 2 below:
[0050]
TABLE 2Deionized Water1.00 LHEPES buffer 100 mmolNaOH 66 mmolNaHCO3 20 mmolNaCl 68 mmolKCl 7.0 mmolCaCl21.00 mmolChloline Chloride 1 mmolGlucose 12 mmolLithium Lactate 4 mmolMIT biocide 2.0 mmolFD&C Blue No. 10.01 gFD&C Yellow No. 50.03 gPEG (MW 2000) 130 gDextran (MW 10,000) 100 gEthylene Glycol 70 g
[0051]To predict the room temperature stability of the solution, accelerated stability studies were performed as described below.
[0052]The reference solution was introduced to a blood analyzer containing an electrode card equipped with sensors to detect pH, carbon dioxide (CO2), oxygen (O2), sodium (Na), potassium (K), calcium (Ca), glucose (Glu), lactate (Lac), and hematocrit (Hct). Twelve hematocrit values were obtained, along with twelve concentration values for each analyte. The average of these values is reported in the time=0 row of FIG. 4.
[0053]Aliquots of the solution were stored at 5° C., 25° C., ...
example 3
[0057]A reference solution was formulated according to Table 3 below:
[0058]
TABLE 3Deionized Water1.00 LHEPES buffer 100 mmolNaOH 44 mmolNaHCO3 20 mmolNaCl 58 mmolKCl 3.0 mmolCaCl22.00 mmolChloline Chloride 1 mmolGlucose 3 mmolLithium Lactate 0.8 mmolMIT biocide 2.0 mmolFD&C Blue No. 10.05 gPEG (MW 2000) 90 gDextran (MW 10,000) 60 g
[0059]Accelerated stability studies on the solution were conducted as described above. First, aliquots of the solution stored at 5° C. and 25° C. were tested as above at 4, 6, and 12 weeks. The results of this study are summarized in FIG. 5. After 12 weeks, no appreciable change in hematocrit and analyte concentration values were recorded, so the test was halted. New aliquots of the solution were placed in ampoules and pasteurized. An accelerated stability study was performed, as described above, on aliquots stored at 5° C., 35° C., and 45° C. After 4 weeks, no appreciable change in hematocrit and analyte concentration values were recorded, as shown...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com