Prevention and treatment of androgen-deprivation induced osteoporosis
a technology of androgen deprivation and osteoporosis, which is applied in the field of prevention and treatment of androgen deprivation induced osteoporosis, can solve the problems of osteoporosis, androgen deprivation therapy is fraught with significant side effects, and achieves the effect of safe and effective treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Toremifene on Bone Turnover in Human Male Subjects
[0094]In a Phase IIa clinical trial to determine whether Toremifene has chemopreventive activity against prostate cancer, 18 men with high-grade prostatic intraepithelial neoplasia (HGPIN) were treated with 60 mg / d of Toremifene for 4 months. At Day 120 there was a significant reduction from baseline in serum calcium (mean −0.12, p=0.005) and at both day 60 and day 120, alkaline phosphatase was significantly decreased compared to baseline (mean=−18.7 at Day 60 and −21.0 at Day 120, and p<0.001 for both visits).
[0095]These clinical data demonstrate that the anti-estrogen Toremifene showed estrogenic effects on bone favorably affecting bone turnover markers in men.
example 2
Effect of Toremifene on Bone in Male Rats
[0096]The test article, positive control and placebo were delivered by ALZA pumps manufactured by Durect Corporation (Cupertino, Calif.). Pumps were implanted in a subcutaneous pocket using appropriate surgical technique. The pumps employed in this study deliver a continuous rate of drug over a 30-day period with Toremifene formulated to release 1.8 mg / day (2 mL pump) and 17-β-Estradiol (positive control) released at 70 ug / day. Data provided by the manufacturer of the pumps validates the constant rate of drug delivery over a 28-day period, and suggests that the constant rate can be expected for several additional days. Animals were anesthetized and pump replacement was performed for each dosage group on days 31, 61, and 91 to provide drug administration over a 120-day period. Every animal on study had a pellet implanted to control for potential confounding variables associated with surgery for implantation.
[00...
example 3
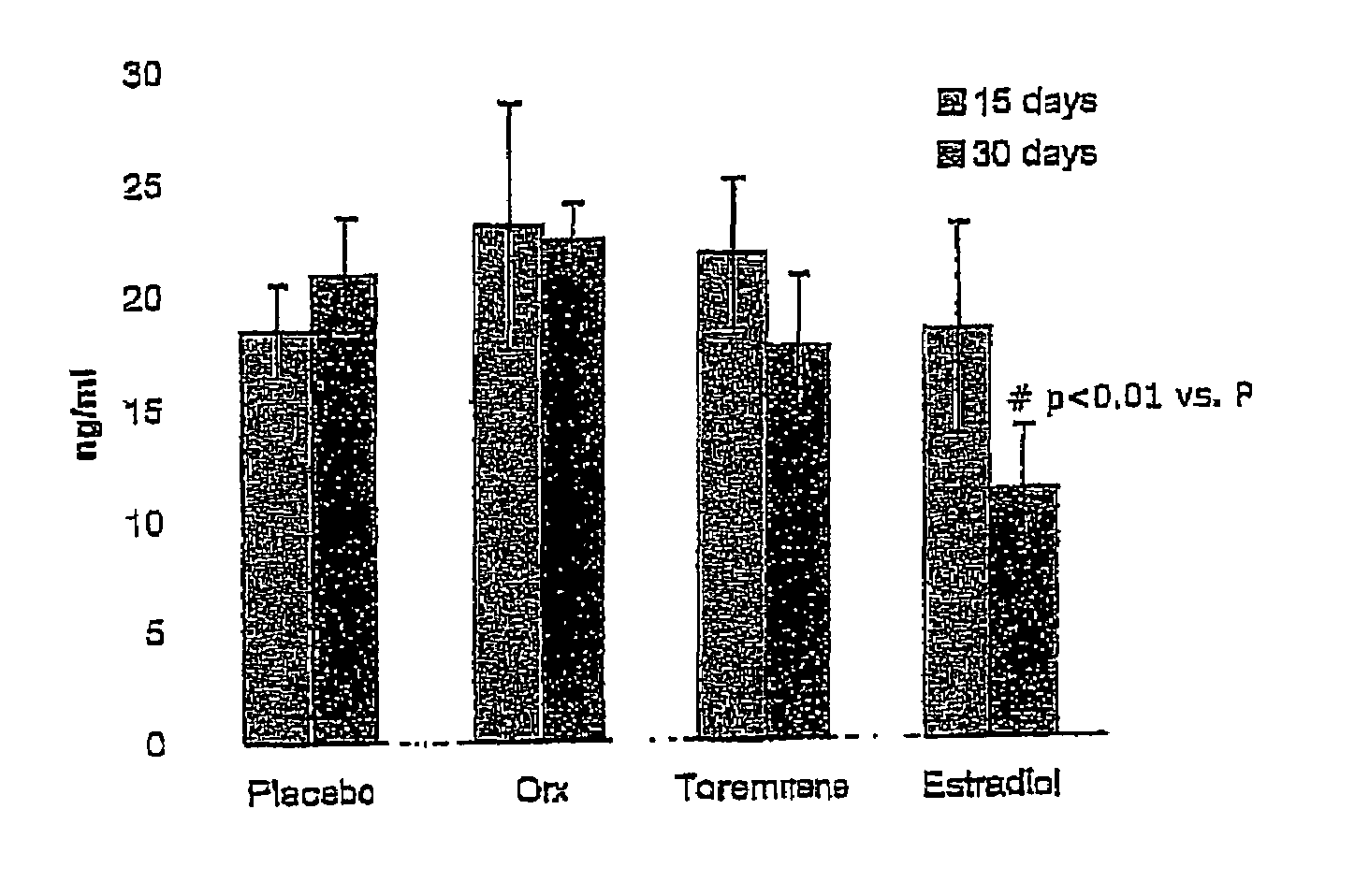
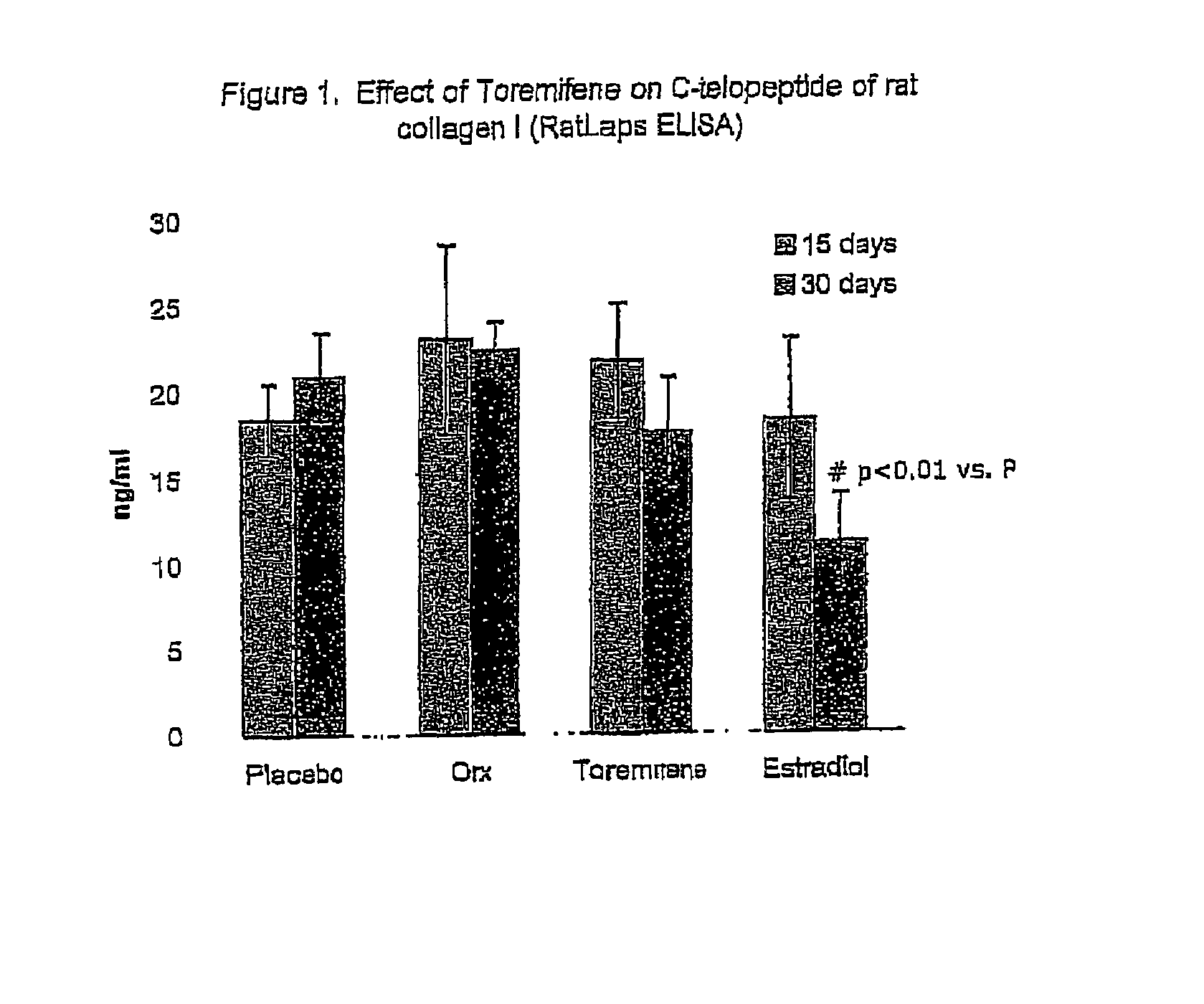
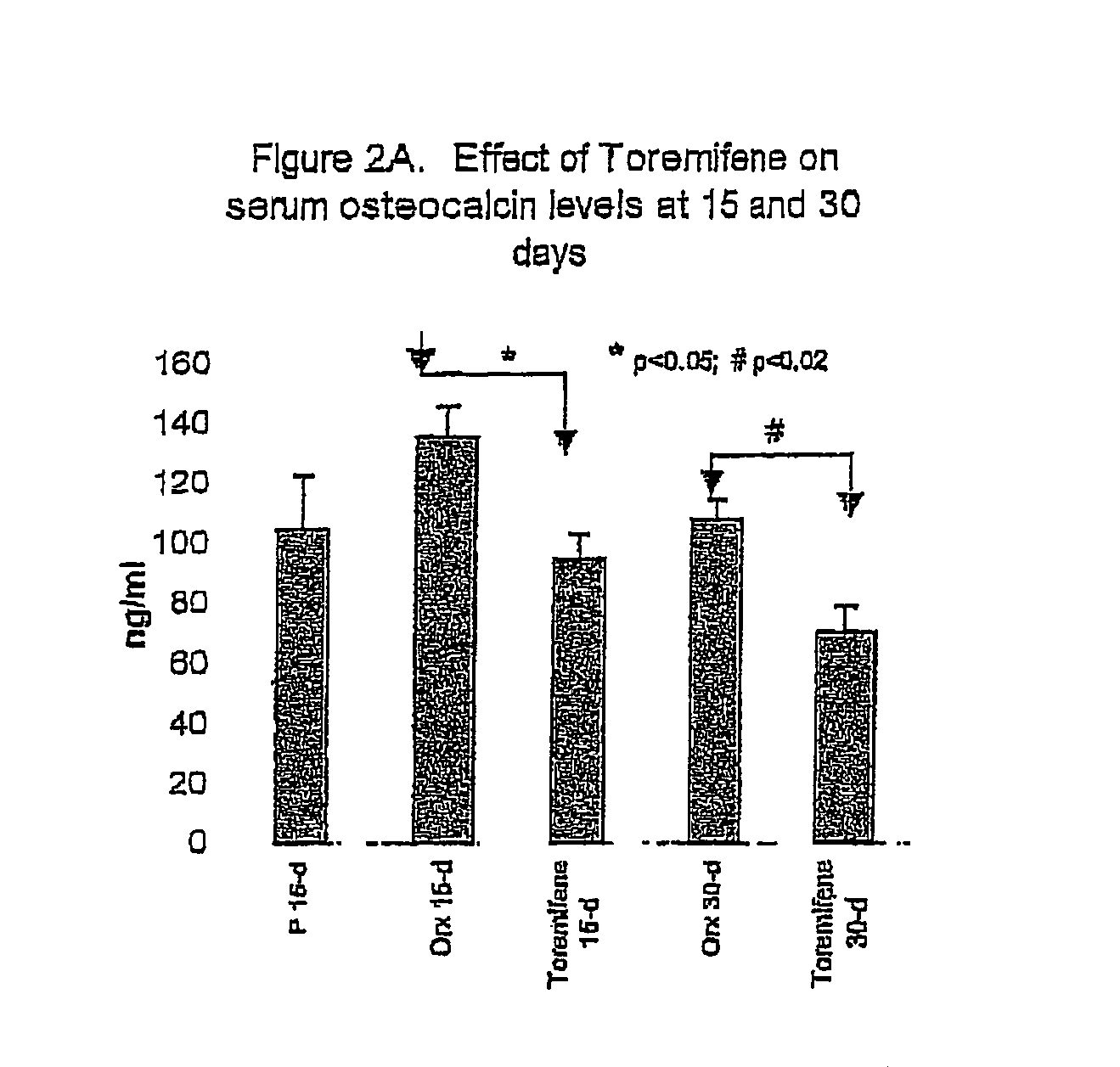
Effect of Toremifene on Bone Density and Serum Markers for Bone Remodeling in Male Rats
[0111]The purpose of this study is to determine whether administration of Toremifene to mature male rats is bone sparing as can presently be measured by the levels of bone-specific serum markers that indicate bone resorption and formation (where 17-β-Estradiol is used as a positive control). The effect of Toremifene (and 17-β-Estradiol) on androgen deprivation-induced bone loss was also determined through bone density and mechanical strength testing.
[0112]The model used herein is an orchidectomy model, which is an experimental model used to mimic the type of androgen deprivation that would be caused by, for example, LHRH agonist therapy in prostate cancer.
Materials and Methods
Study Design
[0113]Male Sprague-Dawley rats (Harlan Sprague Dawley) were placed on study at 14-weeks of age. They were randomized and divided into five treatment groups: vehicle only (placebo, or P) after sham operation, vehic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| bone mineral density | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com