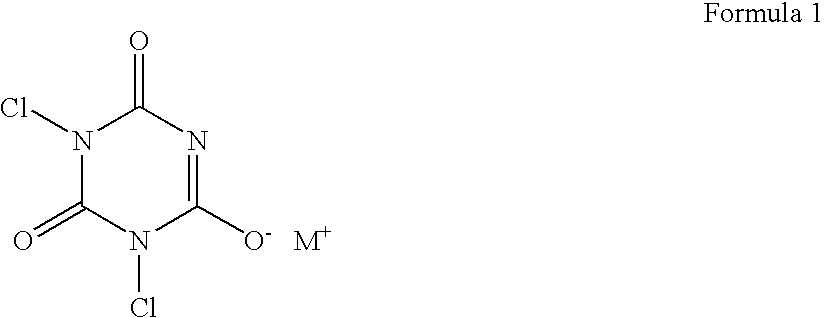Multi-functional oxidizing composition
a multi-functional, oxidizing technology, applied in the direction of biocide, water/sewage treatment by oxidation, treatment water, etc., can solve the problems of chemical instability of mixtures, reduced chlorine sanitizer efficiency, and cloudy and cloudy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0065]OXONE monopersulfate compound (180 g, 90 wt. %) and anhydrous sodium dichloro-s-triazinetrione (20 g, 10 wt. %) were dry blended in a 250-ml high-density poly(propylene (HDPE) bottle. The mixture was placed on a laboratory roll mill for two hours to ensure complete blending of the components. The blended product was then divided into two portions. One portion was stored at ambient conditions (22+ / −2° C., 55+ / −5% relative humidity [RH]); the other in a humid oven at 50+ / −2° C., 80+ / −5% RH denoted as “accelerated aging”). Both portions were stored in sealed screw-cap HDPE bottles. Both samples were monitored on a weekly basis for one month to assess physical integrity and active oxidant loss. The sample at ambient storage conditions remained free flowing, showed no loss in active oxidant concentration, and served as a control. The results for the ‘humid’ oven sample are shown in Table 2 below. It can be seen that even under accelerated aging, the blend remained free flowing, and...
examples 2-12
, Comparative Examples A-E
[0066]Examples 2-12 and Comparative Examples A-E were prepared as described in Example 1 with the component proportions in weight percent as listed in Table 2. As in Example 1, all of the samples at ambient temperature and relative humidity remained free flowing, showed excellent active oxidant stability, and served as control samples. The results for the corresponding accelerated aging storage samples are given in Table 2. It can be seen that Examples 2-12 showed excellent flow properties, low odor, and excellent active oxidant retention after one month at high temperature and 80% relative humidity.
[0067]Comparative Examples A-C, having the compositions defined in Table 2, became caked, exhibited dangerous and malodorous chlorine gas generation, and showed significant loss in active oxidant content. Control C was so unstable from the standpoint of chlorine gas generation that it could not be stored safely at 50° C. for more than a few days. Comparative Exa...
examples 13 and 14
, Comparative Examples C. C′, D, and D′
[0070]Example 13 and Comparative Examples C and D were prepared as described in Example 1 with the component proportions listed in Table 3. The thermal stabilities of these Examples were determined using accelerated rate calorimetry (ARC, see test Method above). These data are presented in Table 3 for three dry samples (13, C, and D) and three corresponding samples where 3 percent by weight water, based on the weight of the dry sample, was added to the sample prior to heating (14, C′, and D′). In this method, the sample was slowly heated under adiabatic conditions through the temperature range in which the sample shows exothermic properties. In Table 3, “Tinitial” represents the temperature at which self-heating begins. The “Max SHR” represents the maximum self-heat rate. This is the maximum slope achieved in the temperature versus time data plot. Finally, “Total Heat” is the total heat evolved as a result of thermal decomposition, i.e., a meas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
| RH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com