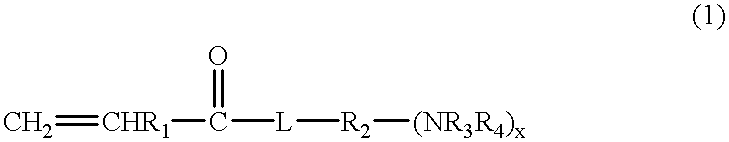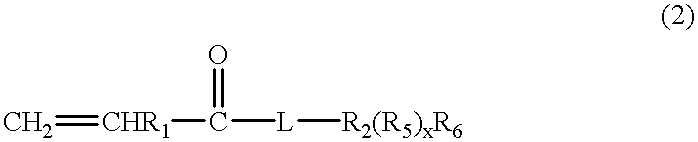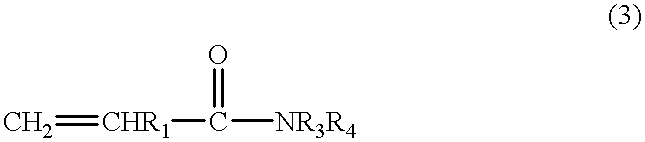Partially crosslinked microspheres
a microsphere and adhesive technology, applied in the field of microsphere adhesives, can solve the problems that the control of the solvent soluble portion of the microsphere to produce a stable repositionable microsphere adhesive with enhanced adhesion has not been considered, and achieves the effects of improved adhesion, easy removal from applied surfaces, and high adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
To a two liter, three necked flask equipped with a thermometer mechanical stirrer and nitrogen inlet tube was charged 597.5 gm of deionized water, 35 gm of a 10% solids solution of Stepanol AMV (trade name for a 28% solids solution of ammonium lauryl sulfate commercially available from Stepan Company), 17.5 gm of a 10% solution of Goodrite K702 (trade name for a 25% solids solution of polyacrylic acid, 240,000 weight average molecular weight, commercially available from B.F. Goodrich Company) which had been neutralized to a pH of 7.0 with concentrated ammonium hydroxide. To this solution was added 350 gm of isooctyl acrylate and 1.1 gm of Perkadox 16N (trade name for a 95% active bis (4-tert-butylcyclohexyl) peroxydicarbonate initiator commercially available from AKZO Chemicals Inc.), and 0.14 gm (0.04% by weight of the monomer) of dodecanethiol (a chain transfer agent commercially available from Aldrich Chemical Company). The agitation rate was set at 410 revolutions per minute (RP...
example 2-6
The examples were prepared according to the procedure described in Example 1 except that the amount of dodecanethiol was changed and the amount and type of comonomer was changed. The formulations and test results are summarized in Tables 1 and 2 below.
TABLE 1 % Solvent Particle Soluble Example Comonomer % Dodecanethiol Size (.mu.m) Portion 1 None 0.04 54 57 2 1% Hydroxy 0.04 53 71 Ethyl Methacryl- ate 3 1% Acrylic Acid 0.03 43 83 4 0.5% Acrylic 0.02 34 92 Acid 5 1% N Vinyl 0.02 42 65 Pyrrolidone 6 1% Acrylamide 0.03 35 80
TABLE 1 % Solvent Particle Soluble Example Comonomer % Dodecanethiol Size (.mu.m) Portion 1 None 0.04 54 57 2 1% Hydroxy 0.04 53 71 Ethyl Methacryl- ate 3 1% Acrylic Acid 0.03 43 83 4 0.5% Acrylic 0.02 34 92 Acid 5 1% N Vinyl 0.02 42 65 Pyrrolidone 6 1% Acrylamide 0.03 35 80
example 9
To a two liter, three-necked flask equipped with a thermometer, mechanical stirrer and nitrogen inlet tube was charged 158 grams of a 0.7% solids solution of Standapol A (trade for a 28% solids solution of ammonium lauryl sulfate commercially available from Henkel Corporation), 158 grams of 1.6% solution of acrylic acid in deionized water, and enough concentrated ammonium hydroxide to neutralize the solution to pH 7. To this solution was added a solution comprised of 100 grams of isooctyl acrylate, 5 grams of heptane and 0.488 grams of Lucidol 70 (trade name for a 70% active benzoyl peroxide initiator commercially available from Pennwalt Corporation). The agitation rate was set to 400 rpm. The reaction mixture was heated to 65.degree. C., purged with nitrogen and reacted at 65.degree. C. for 8 hours. The reaction was then cooled and filtered through cheesecloth. The particle size was 41 .mu.m and the % solvent soluble portion was 59%.
This example showed that solvent could be used as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com