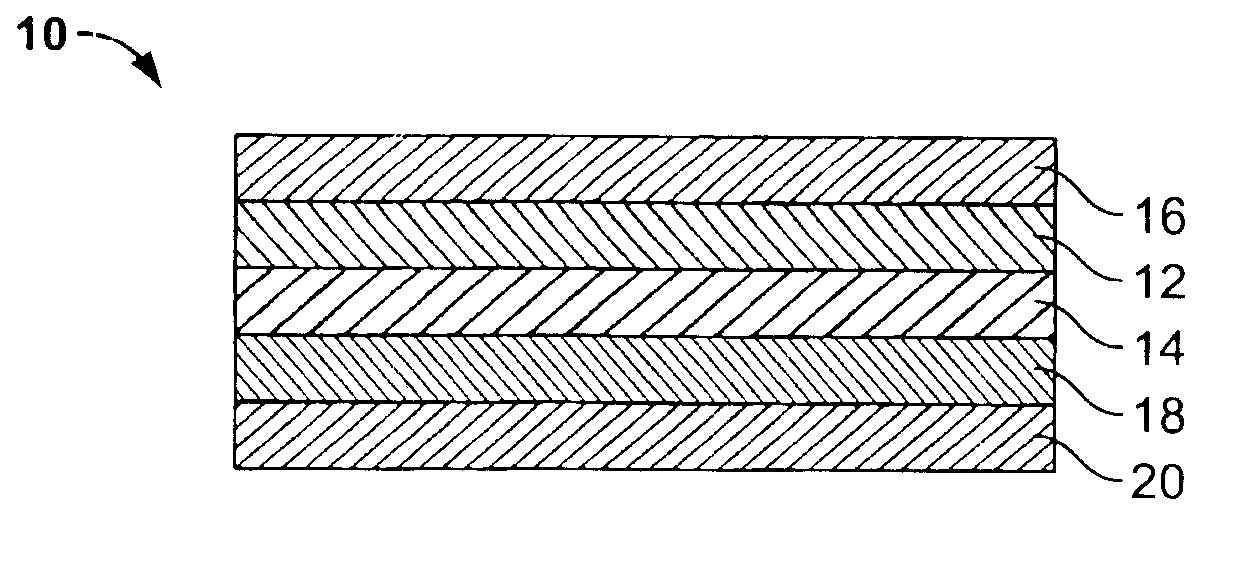
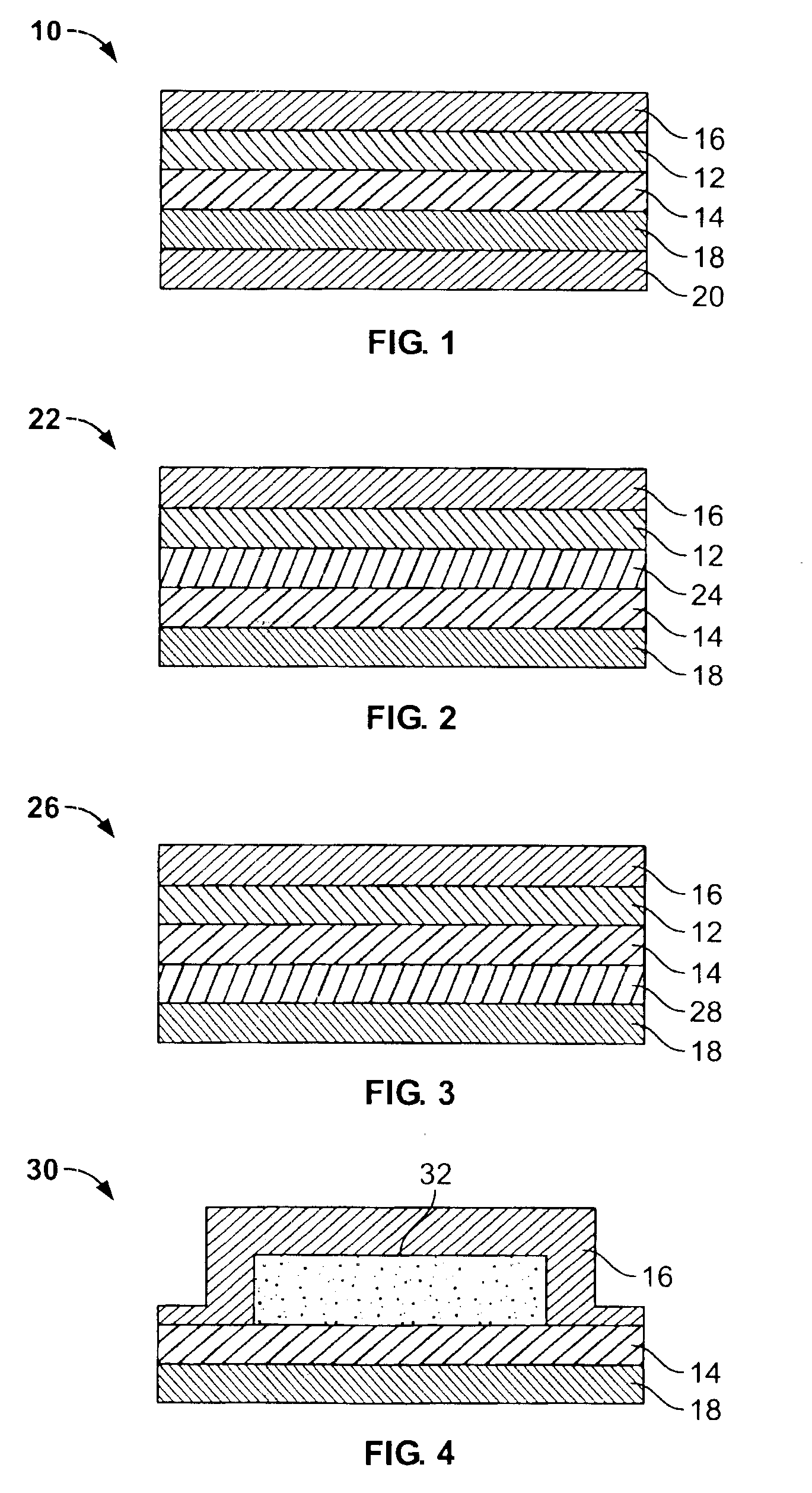
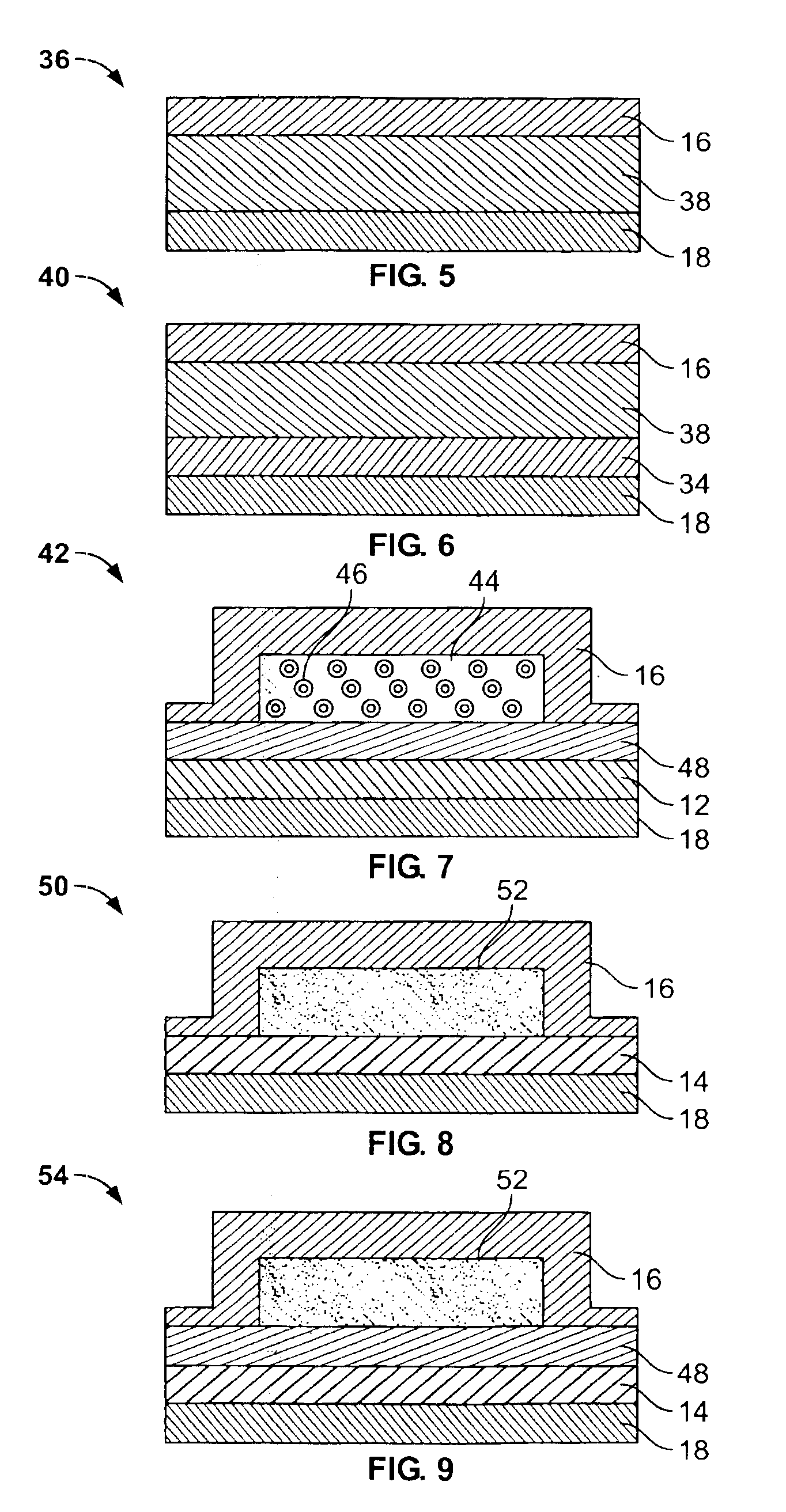
Transdermal drug delivery device
a technology of drug delivery and transdermal injection, which is applied in the direction of prosthesis, bandages, dressings, etc., can solve the problems of unsuitable, delayed onset of therapeutic effect, and inability to possess the capability of diffusional delivery devices known to the art, and achieve the effect of delayed onset of drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0064]A test sample was designed to deliver nicotine according to this invention. Nicotine which is transdermally administered to facilitate breaking the tobacco habit, is especially suited to illustrate this invention. Nicotine in its active form tends to degrade adhesives, especially those which are silicone based. Also, nicotine delivered in a sudden burst may irritate the skin. Therefore, use of this invention to deliver nicotine provides a system with an improved shelf-life by storing the nicotine in its salt form, and further provides elimination of the initial burst of drug by storing nicotine in a form which is not readily absorbed through mucous membrane and intact skin.
[0065]The drug reservoir was a polymeric matrix comprised of 60% nicotine tartrate and 40% EVA 40 carrier, and was about 6.5 mils thick. The activating layer was about 3.5 mils thick and was a polymeric matrix comprised of 60% Na2CO3 and 40% EVA 40 carrier. Sodium carbonate provides an aqueous solution which...
example ii
[0070]A system was designed according to this invention and is illustrated in FIG. 76. The backing was standard Medpar® and the system had the storage suitable form of the drug and the activating agent (base) mixed in the same reservoir. The drug reservoir was comprised of 42% nicotine tartrate, 18% Na2CO3 and 40% EVA 40 carrier. The system had a PVA rate controlling membrane about 1.5 mils thick and had a silicone adhesive layer.
[0071]The release rate of nicotine from this system at 35° C. in a release medium of water, is presented in the following table:
[0072]
TABLE 1Average Release Rate,Time, hrsμg / cm2 / hr272.64429.55665.84852.6013112.51
[0073]As is evidenced from the data presented, this system provides the desired delayed onset where the drug release rate during the first 8 hours is minimal and gradually increases to a significant level at 13 hours.
example iii
[0074]A test sample was designed to deliver nicotine. The drug reservoir was a polymeric matrix comprised of 40% nicotine tartrate, 50% EVA 40 carrier and 10% NaCl, and was about 8 mils thick. The activating layer was about 3.5 mils thick and was a polymeric matrix comprised of 60% Na2CO3 and 40% EVA 40 carrier.
[0075]The release rate was measured using a 1.5 mil Hytrel® membrane and is graphically illustrated in FIG. 11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com