Aminoindan derivatives
a technology of aminoindan and derivatives, applied in the field of compounds, can solve the problem of weak active compound only
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Acetylcholinesterase Inhibition in Mice
[0090]1.1 In vitro measurement of Acetylcholinesterase (AChE) Inhibition
[0091]Human erythrocyte acetylcholinesterase (type XIII, Sigma Israel), was prepared in a stock solution of 1 U / ml, containing Triton (1%) and bovine serum albumin (0.05%) in phosphate buffer (pH 8). The enzyme (0.05U) was incubated with 3-5 different concentrations of test compound (in triplicate) for periods of from 15 to 60 minutes at 37° C. The substrate acetylthiocholine (0.075M) and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 0.01M) were then added and the rate of hydrolysis of the substrate which yields a yellow product monitored spectrophotomerically at 412 nM (Ellman et al., Biochem Pharmacol. (1961) 7: 88-95). The percentage inhibition of AChE by each concentration of drug is calculated by comparison with that of enzyme in the absence of drug. The concentration of each drug that inhibits AChE by 50% (IC50) at the time of peak activity was calculated and is given i...
example 2
[0096]2.1 Inhibition of MAC activity in vitro
[0097]The MAO enzyme source was a homogenate of rat brain in 0.3M sucrose, which was centrifuged at 600 g for 15 minutes. The supernatant was diluted appropriately in 0.05M phosphate buffer, and pre-incubated with serial dilutions of test compounds for 20 minutes at 37° C. 14C-Labeled substrates (2-phenylethylamine, hereinafter PEA; 5-hydroxytryptamine, hereinafter 5-HT) were then added, and the incubation continued for a further 20 minutes (PEA), or 30-45 minutes (5-HT). Substrate concentrations used were 50 μM (PEA) and 1 mM (5-HT). In the case of PEA, enzyme concentration was chosen so that not more than 10% of the substrate was metabolized during the course of the reaction. Deaminated products were extracted into toluene-ethyl acetate (1:1 v / v) containing 0.6% (w / v) 2,5-diphenyloxazole (ppo) prior to determination by liquid scintillation counting. Radioactivity n the eluate indicates the production of neutral and acidic metabolites fo...
example 3
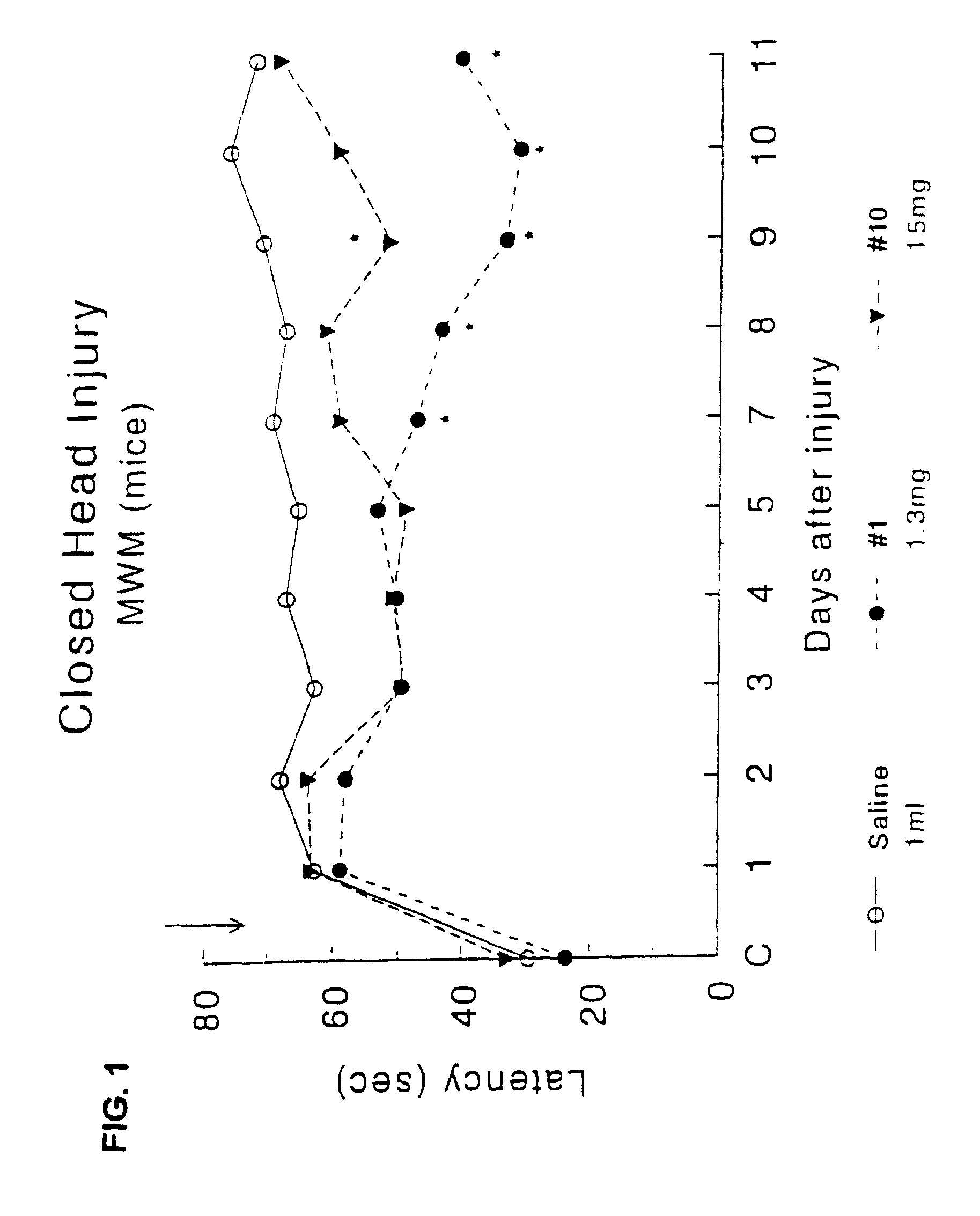
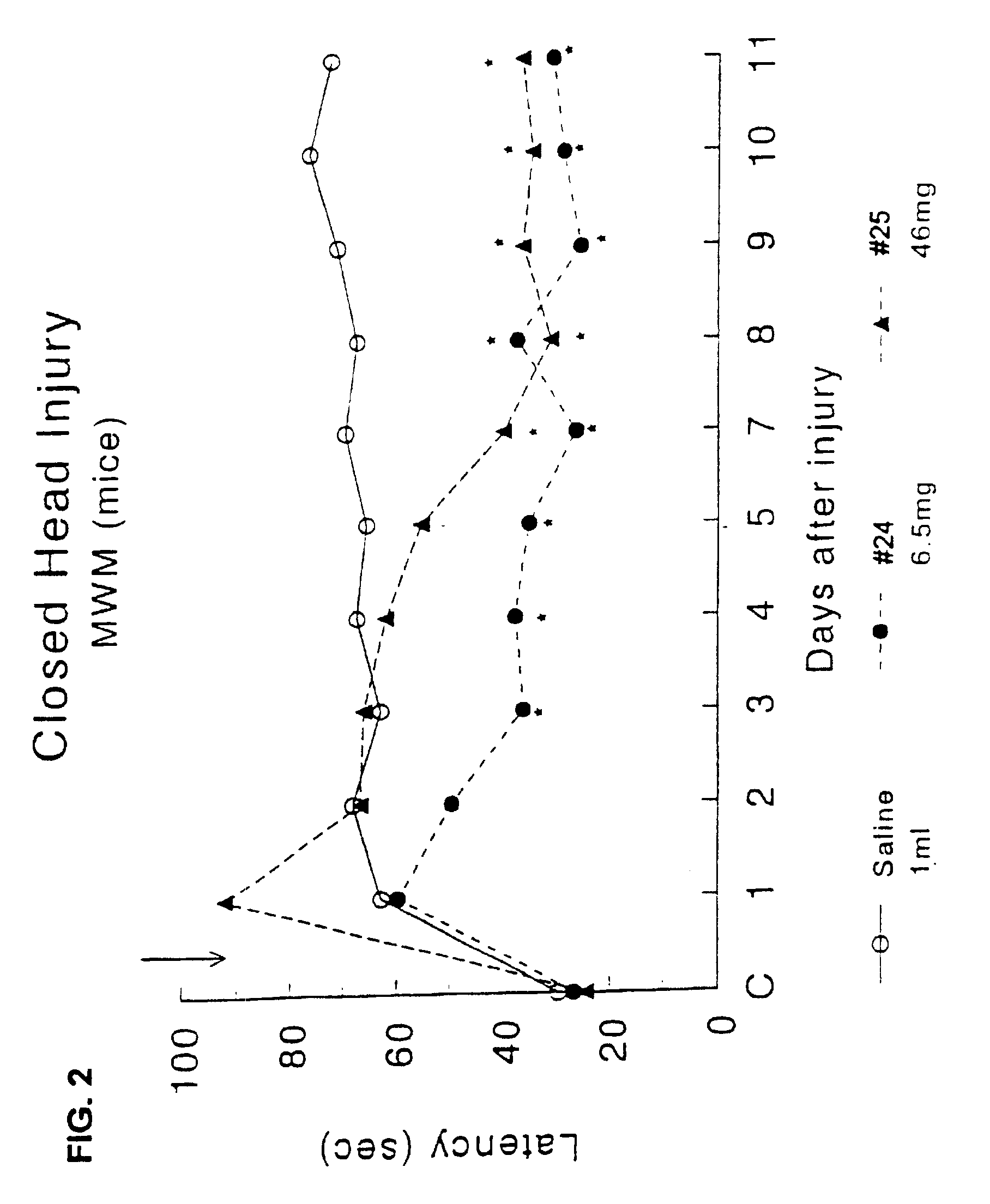
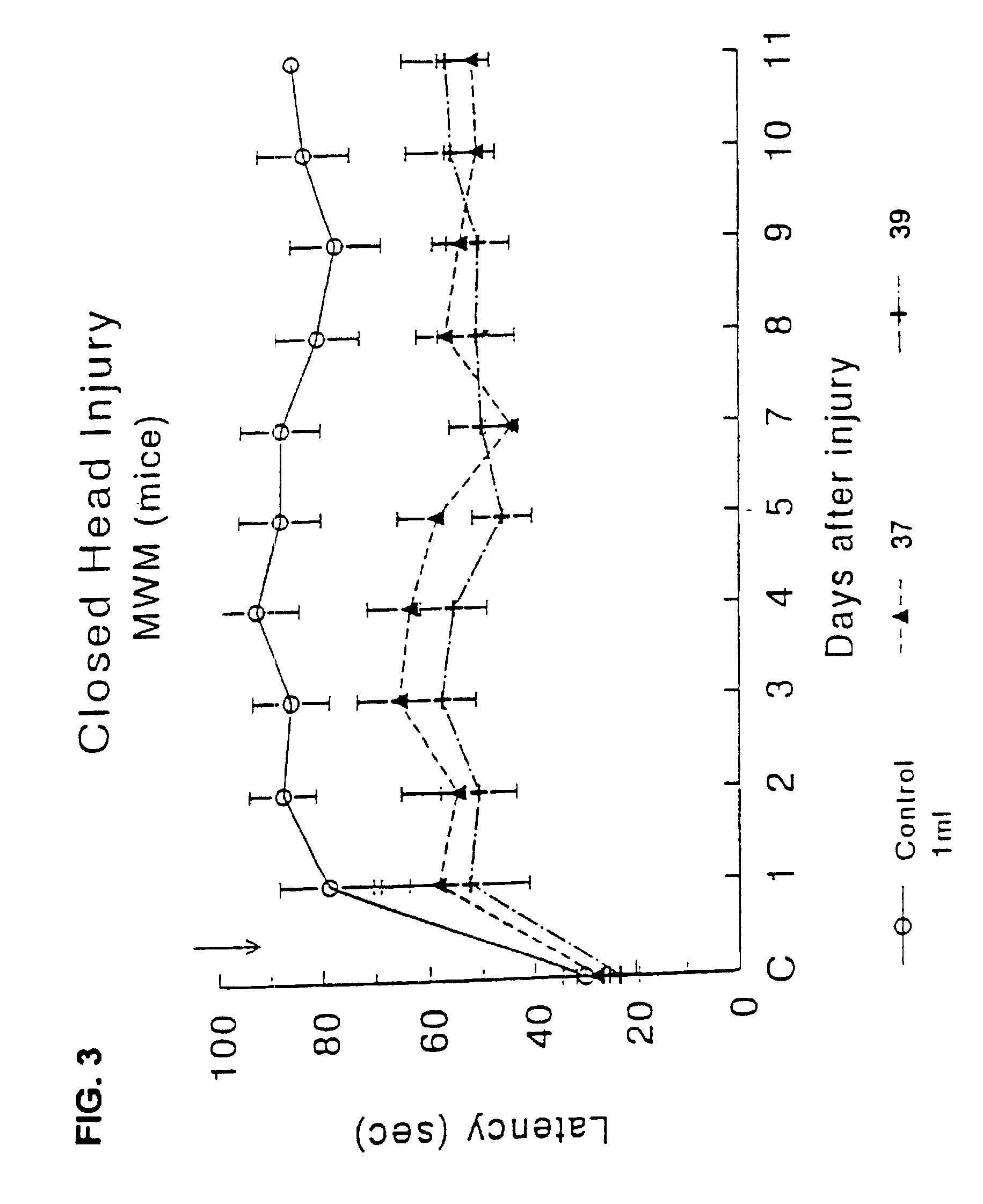
Effect of Drug Treatment Following Closed Head Injury (CHI) in Mice
[0105]The procedure for closed head injury followed was as described for rats in Shohami, et al. (J Neurotrauma (1993) 10 (2): 109-119) with changes as described.
[0106]Animals: Male Sabra mice (Hebrew University strain) weighing 34-40 g were used. They were housed in groups of 10 per cage, in a 12 hr:12 hr light:dark cycle. Food and water were provided ad libitium.
[0107]Trauma was induced under ether anesthesia. A longitudinal incision was performed in the skin covering the skull and the skin retracted to expose the skull. The head was fixed manually at the lower plane of the impact apparatus. A weight of 333 g was delivered by an electric device from a distance of 3 cm to the left hemisphere, 1-2 mm lateral to the midline in the midcoronal plane. Test compounds were injected sub-cutaneously at a dosage corresponding to the ED50 acetylcholinesterase, once 15 min. after CHI.
[0108]3.1 Assessment of Motor Function
[0109]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com