Method of pre-treating sample for measuring saccharified amine and method of measuring saccharified amine
a technology of saccharified amine and pre-treating sample, which is applied in the direction of enzymology, instruments, and detection of post translational modifications, can solve the problems of inability to perform reliable measurement and more hydrogen peroxide formation, and achieve the effect of eliminating influence and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
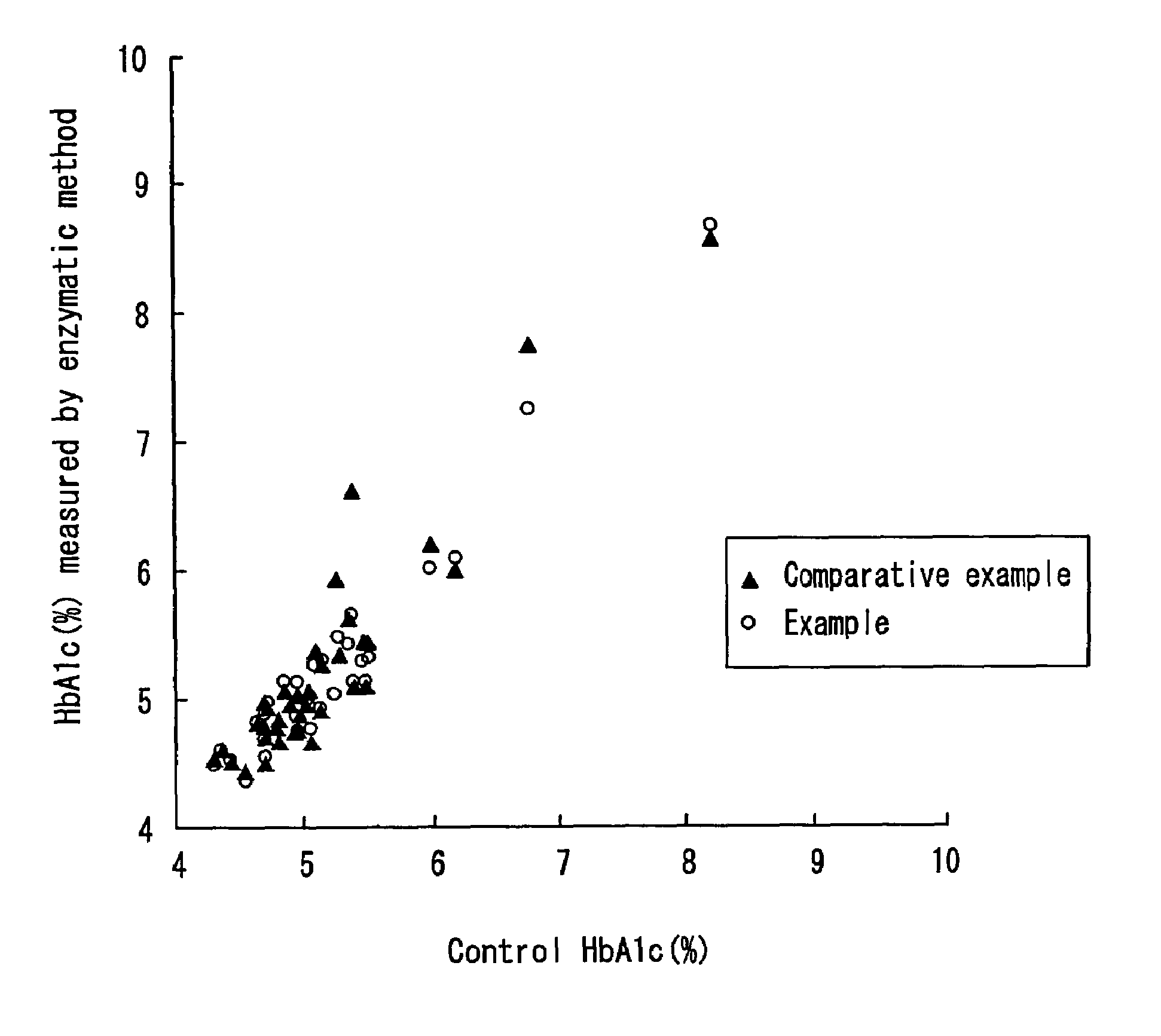
Examples
first embodiment
[0042]The present embodiment is one example of the first method, in which a FAOD-α is used to degrade the glycated amino acid and a FAOD-αS is used to measure the glycated protein.
[0043]First, whole blood is hemolyzed to prepare a hemolyzed sample. The method of causing the hemolysis is not particularly limited, and can be, for example, a method using a surfactant, a method using ultrasonic waves, a method utilizing a difference in osmotic pressure, and a method using a freeze-thawing technique. Among these, the method using a surfactant is preferable because of its simplicity in operation, etc.
[0044]As the surfactant, for example, non-ionic surfactants such as polyoxyethylene-p-t-octylphenyl ether (e.g. Triton series surfactants), polyoxyethylene sorbitan alkyl ester (e.g. Tween series surfactants), polyoxyethylene alkyl ether (e.g. Brij series surfactants), and the like can be used. Specific examples are products named Triton X-100, Tween-20, Brij 35, and the like. The conditions ...
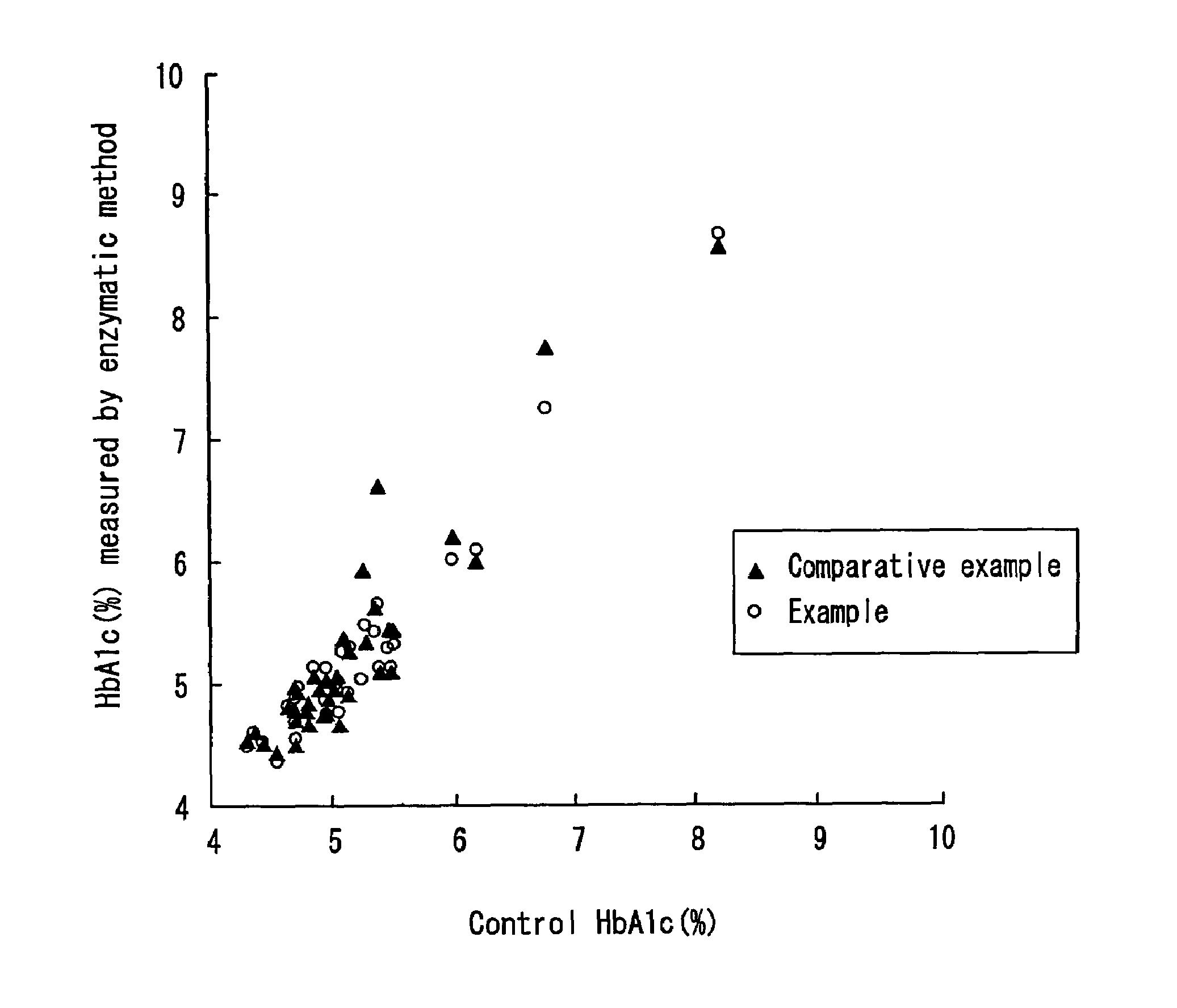
second embodiment
[0064]The present embodiment is one example of the second method in which the same FAOD is used to degrade a glycated amino acid as a non-analyte glycation product and to measure a glycated protein as an analyte. The FAOD used in not particularly limited, and for example, any of a FAOD-α, a FAOD-S, and a FAOD-αS may be used.
[0065]A hemolyzed sample is prepared in the same manner as in the first embodiment, and a degradation FAOD is added to this hemolyzed sample.
[0066]The treatment is carried out, for example, under the conditions as follows: the concentration of the FAOD in the reaction solution in the range from 10 to 5000 U / l, the concentration of the blood cells in the reaction solution in the range from 0.5 to 20 vol %, the reaction temperature in the range from 20° C. to 50° C., the reaction period in the range from 1 minute to 1 hour, and the pH in the range from 6 to 9. This treatment usually is carried out in a buffer, and the same buffers as described above also can be use...
third embodiment
[0077]The present embodiment is an example where the same FAOD is used to degrade a glycated amino acid as a non-analyte glycation product and to measure a glycated protein as an analyte. However, the present embodiment differs from the above-described second embodiment in that it is not always necessary to inactivate a degradation FAOD with a protease. Because of the substrate specificity of enzymes, inactivating a FAOD with a protease can be difficult depending on the combination of the FAOD and protease. A method for measurement according to the present embodiment is effective in such a case. If a degradation FAOD added first reacts with a glycated protein degradation product formed by the treatment with a protease, the accuracy of the measurement cannot be improved. Accordingly, it is important to adjust the ratio of a degradation FAOD to a measurement FAOD added to a sample as described later.
[0078]First, a hemolyzed sample is prepared in the same manner as in the first embodim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com