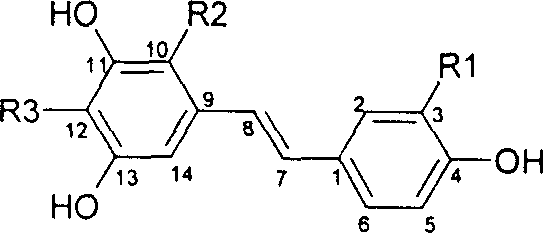
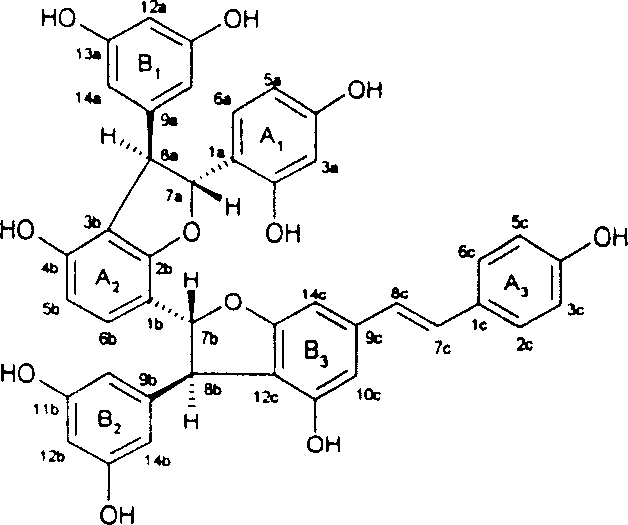
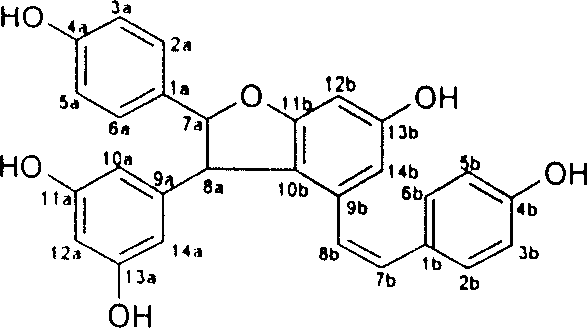
Resveratrol oligo cattail compounds, its manufacturing process, pharmaceutical combination and uses thereof
A technology of drugs and ethanol, applied in the fields of drug combination, antipyretic, organic chemistry, etc., can solve the problems of difficulty, difficult separation of compounds in large quantities, and insufficient depth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1. The preparation of the effective fraction group extract of Dazimaimateng:
[0088] Gnetum Montanum f.megalocarpum (Gnetum Montanum f.megalocarpum) stem 10kg is pulverized, respectively with 5 times the amount, 4 times the amount, 4 times the amount, refluxed with 95% ethanol for 2 hours, extracted 3 times, and concentrated the extract under reduced pressure to obtain a rough extract Cream 874 grams. The crude extract was refluxed with 3 times the amount of petroleum ether to degrease twice, each time for 1 hour, and the degreased extract was refluxed with 3 times the amount of acetone to extract 4 times, each time for 1 hour. Concentrate the acetone-soluble part, mix the sample with 30-60 mesh polyamide according to the multiple of 1:2, pack it into the column at 1:5, and fully elute with 30%-80% ethanol. Collect 30%-80% ethanol and concentrate partly to obtain 44.2g of the effective part extract of Dazimai Mateng.
Embodiment 2
[0089] Example 2. Preparation of gnetumontanin B (gnetumontanin B, 1):
[0090] Dazi bought Gnetum montanum f.megalocarpum stem (10Kg), ground it and extracted it four times with 95% alcohol under reflux, distilled under reduced pressure to remove the solvent, obtained crude extract (870g) Soxhlet extraction, respectively with chloroform, Ethyl acetate, acetone, methanol elution. The ethyl acetate fraction (200g) was further subjected to silica gel column chromatography (140-180 mesh), chloroform-methanol gradient elution (30:1, 20:1, 15:1, 12:1, 10:1, 9:1 , 8:1, 6:1, 5:1, 4:1), divided into eight parts A-H. Part H (44g) was subjected to silica gel column chromatography (140-180 mesh), cyclohexane-acetone gradient elution (1:1, 1:1.5, 1:2, 1:2.5, 1:3, 1:4) Divided into six parts (H 1 -H 6 ). h 4 A portion (0.2 g) was subjected to medium pressure column chromatography, eluting with methanol-water (3.5:6.5) to obtain compound 1 (31 mg).
[0091] Compound 1 is light yellow...
Embodiment 3
[0094] Embodiment 3. Preparation of bis-oxyresveratrol (bis-oxyresveratrol, 7):
[0095] Dazi bought Gnetum montanum f.megalocarpum stem (10Kg), ground it and extracted it four times with 95% alcohol under reflux, distilled under reduced pressure to remove the solvent, obtained crude extract (870g) Soxhlet extraction, respectively with chloroform, Ethyl acetate, acetone, methanol elution. The ethyl acetate fraction (200g) was further subjected to silica gel column chromatography (140-180 mesh), chloroform-methanol gradient elution (30:1, 20:1, 15:1, 12:1, 10:1, 9:1 , 8:1, 6:1, 5:1, 4:1), divided into eight parts A-H. Part H (44g) was subjected to silica gel column chromatography (140-180 mesh), cyclohexane-acetone gradient elution (1:1, 1:1.5, 1:2, 1:2.5, 1:3, 1:4) Divided into six parts (H 1 -H 6 ). h 2 Part (0.23g) was subjected to medium pressure column chromatography, eluting with methanol-water (4:6) to obtain compound 7 (45mg).
[0096] Compound 7 is light yellow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com