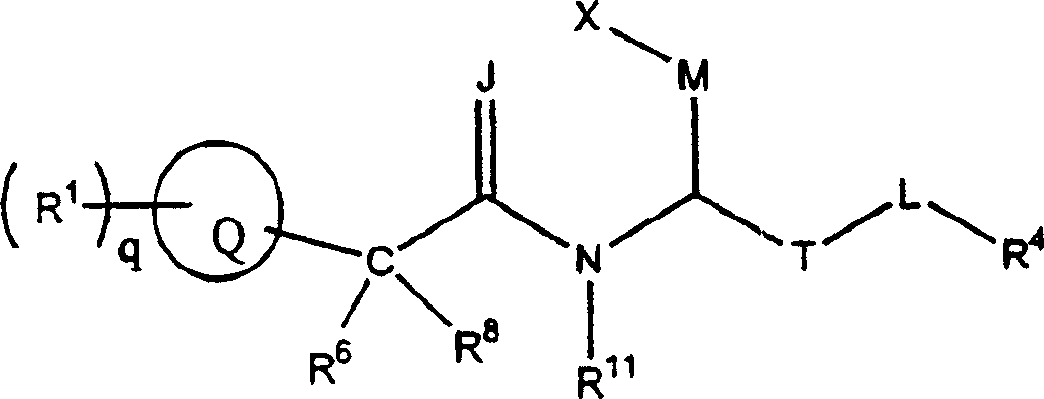
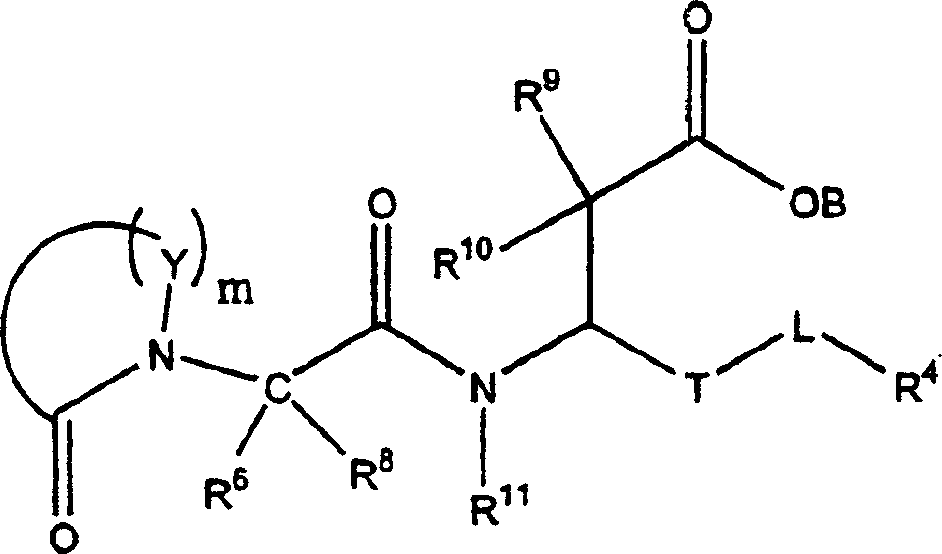
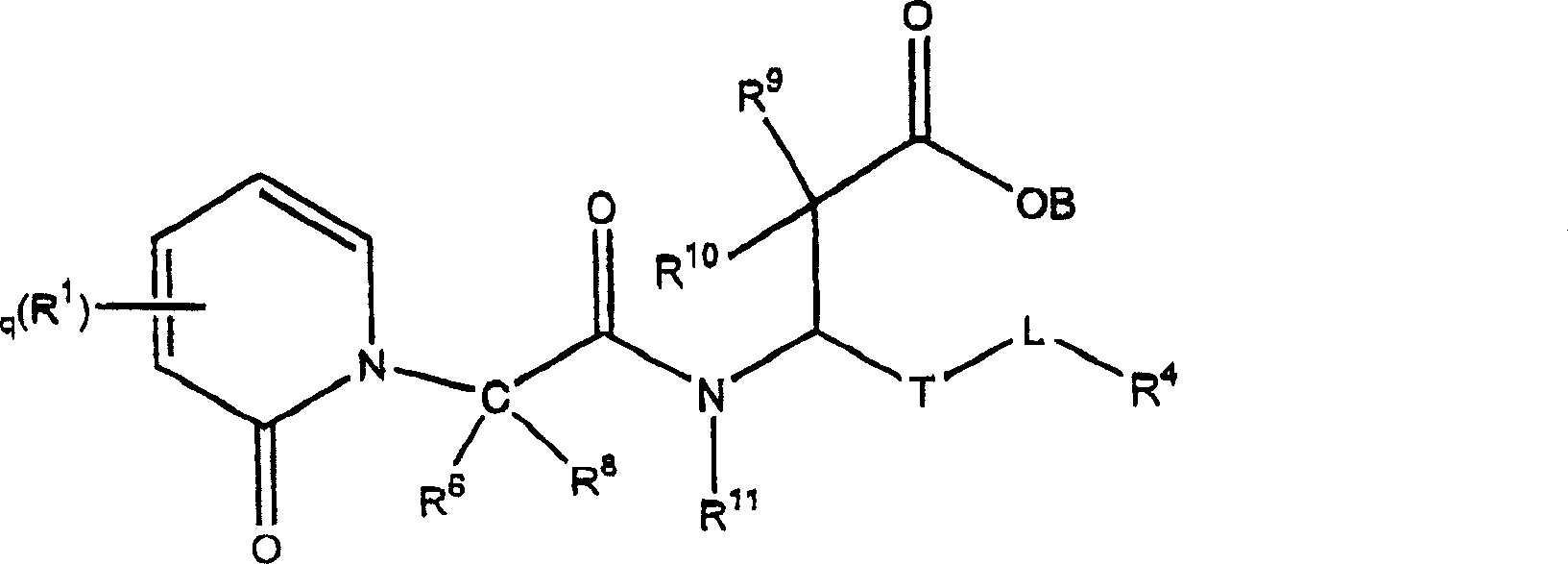
Propanoic acid derivatives that inhibit binding of integrins to their receptors
A technology of derivatives and compounds, applied in the field of preparations for disease states, can solve problems such as uncontrollable leukocyte migration, tissue damage, blood cell influx, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0112]
[0113] Process 4
[0114] Scheme 5, shown below, illustrates the method of Example 13.
[0115]
[0116] Process 5
[0117] Scheme 6, shown below, illustrates the method introduced in Example 14.
[0118]
[0119] Process 6
[0120] Scheme 7, shown below, illustrates the method introduced in Example 15.
[0121]
[0122] Process 7
[0123] Flowchart 8 shown below illustrates the method introduced in Example 16.
[0124]
[0125] Process 8
[0126] Scheme 9, shown below, illustrates the method introduced in Example 17.
[0127]
[0128] Process 9
[0129] Flowchart 10 shown below illustrates the method introduced in Example 18.
[0130]
[0131] Process 10
[0132] Flowchart 11 shown below illustrates the method introduced in Example 19.
[0133]
[0134] Process 11
[0135] Flowchart 12 shown below illustrates the method introduced in Example 20.
[0136]
[0137] Process 12
[0138] Flowchart 13 shown below illustrates the meth...
Embodiment 1
[0177] Compound 8(3S)-3-(1,3-benzodioxol-5-yl)-3-((2R,S)-2-(3-benzyl- 5-methyl-2-oxo-1(2H)-pyridyl)hexanoylamino)propanoic acid.
[0178]
[0179] The structures of the compounds indicated by numbers in this example are shown in Scheme 1 above.
[0180] step 1 : A solution of 540 mg of methyl 2-aminocaproic acid hydrochloride 1 in 20 ml of dichloromethane was washed with excess saturated sodium bicarbonate solution. The organic layer was separated, dried over magnesium sulfate, and concentrated in vacuo to afford 365 mg of methyl 2-aminocaproate as a colorless oil. This material was mixed with 5 ml benzene, 0.28 ml propionaldehyde and excess magnesium sulfate. After stirring for 15 minutes, the reaction mixture was filtered and concentrated in vacuo to afford 420 mg of compound 2 as a colorless oil. Compound 2 was used directly without further purification.
[0181] step 2 : Under a positive nitrogen atmosphere, add 0.80 ml of triethylamine and 964 mg of 3-phenylpro...
Embodiment 2
[0190] Synthesize the compound 12(3S)-3-((2R,S)-2-(3-benzyl-5-methyl-2-oxo-1(2H)-pyridine shown below according to the method of Example 1 Base) hexanoylamino)-3-(2,3-dihydro-1-benzofuran-5-yl)propanoic acid,
[0191]
[0192] The exception in the method is to replace compound 6 in step 5 with compound A shown below.
[0193]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com