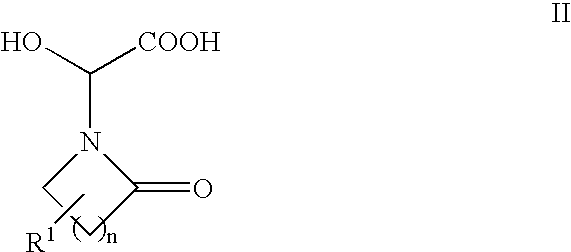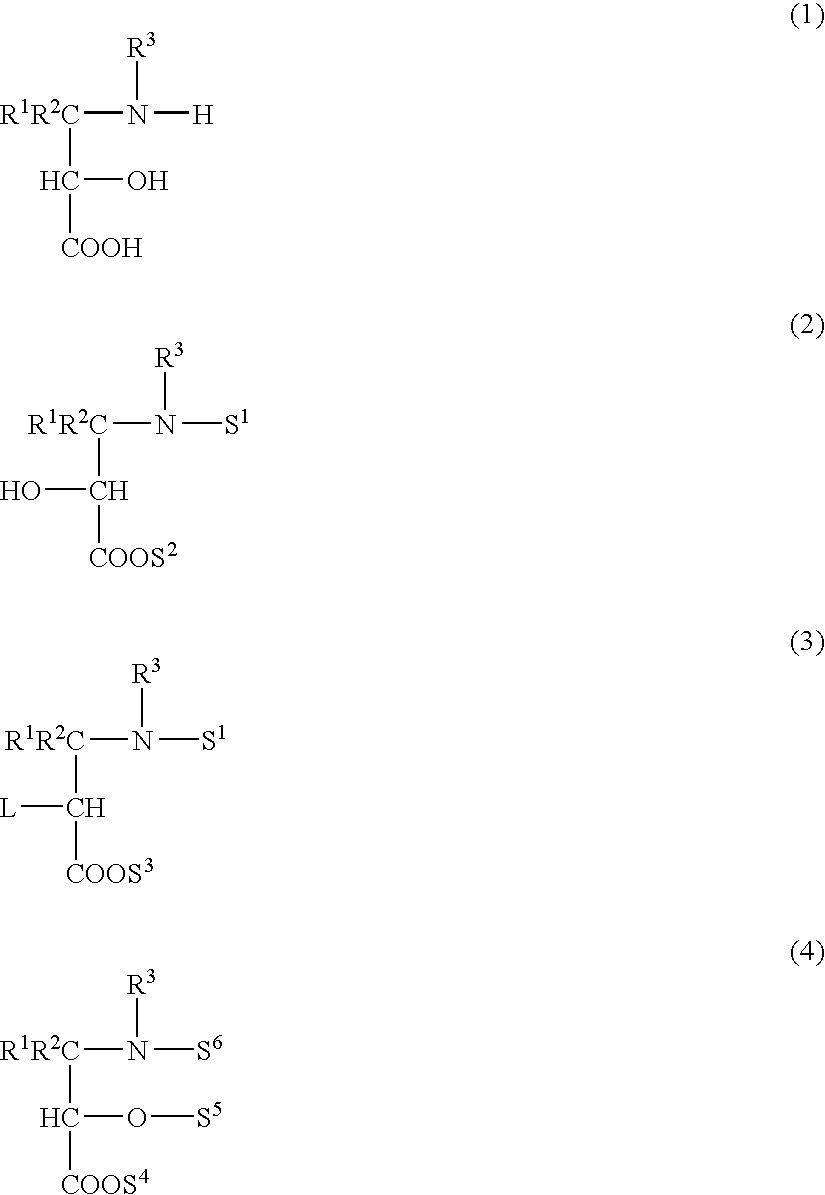Patents
Literature
35 results about "Propanoic Acid Derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
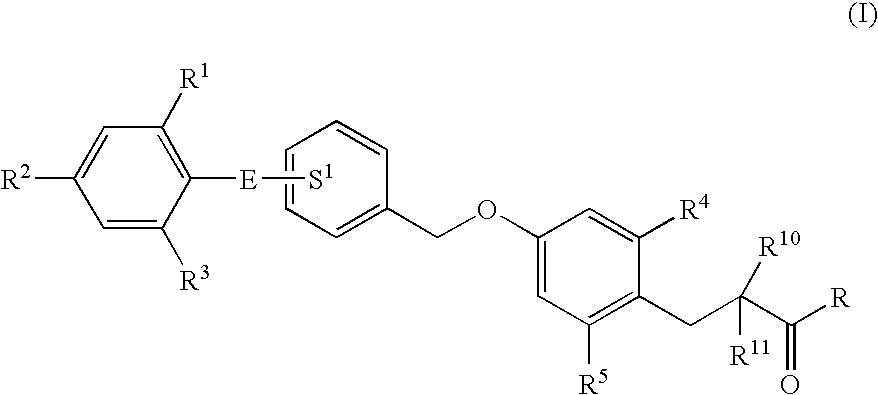
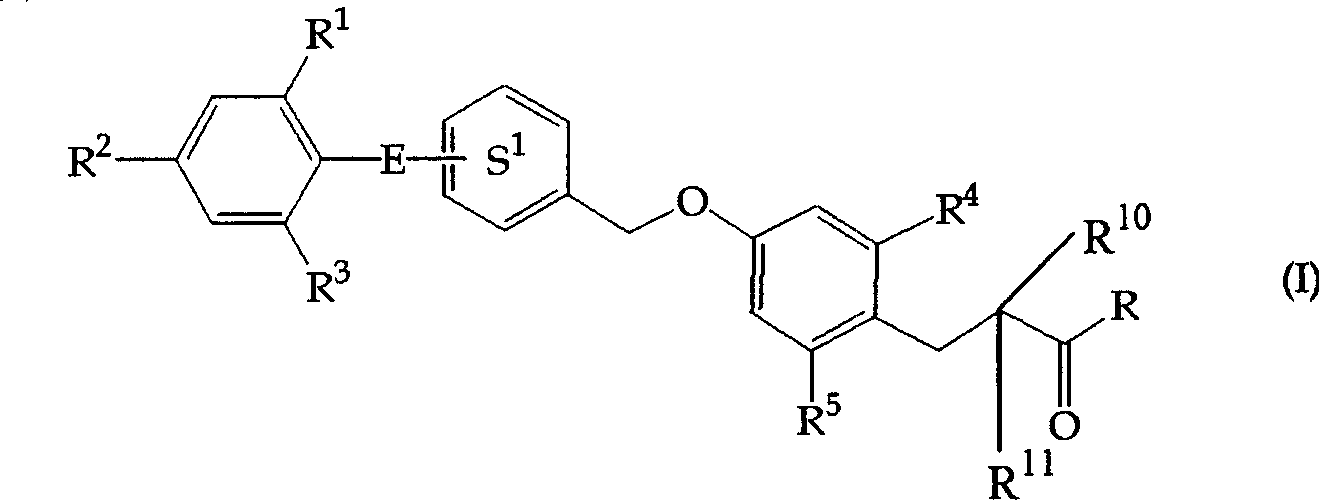
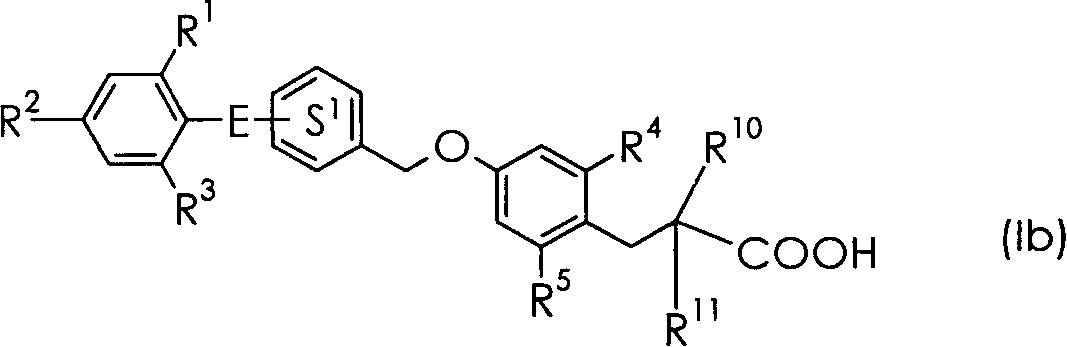
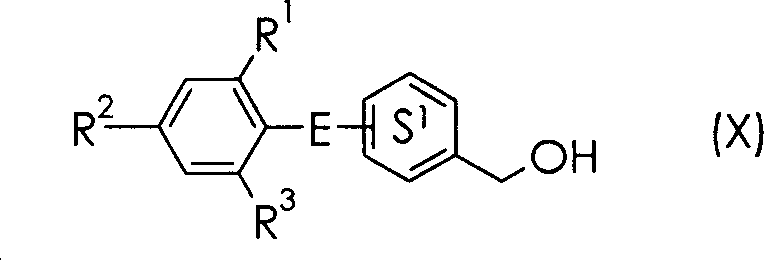
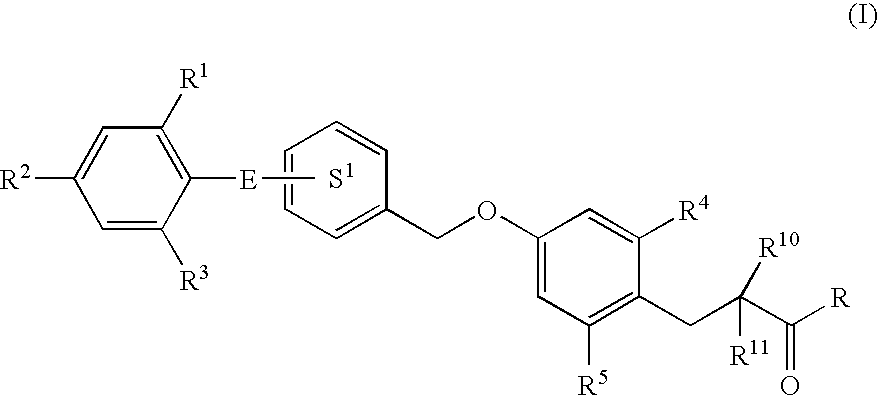
3-(4-Benzyloxyphenyl) propanoic acid derivatives
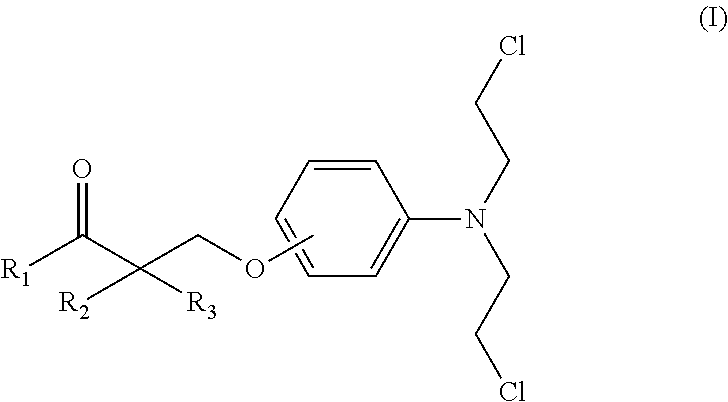
InactiveUS20070149608A1Superior agonist activityEasy to useBiocideGroup 4/14 element organic compoundsDiabetes mellitusMammal
The present invention provides a novel compound represented by the formula (I) wherein each symbol is as defined in the specification, a salt thereof and a prodrug thereof having a superior GPR40 receptor function modulating action, which can be used as an insulin secretagogue, an agent for the prophylaxis or treatment of diabetes and the like. They unexpectedly show superior GPR40 receptor agonist activity, and also show superior properties as a pharmaceutical product, such as stability and the like. Thus, they can be safe and useful pharmaceutical agents for the prophylaxis or treatment of GPR40 receptor related diseases in mammals.
Owner:TAKEDA PHARMA CO LTD
3-(4-benzyloxyphenyl)propanoic acid derivative
InactiveCN1922165AModulation of high GPR40 receptor functionPrevent diabetesOrganic chemistryDiabetes mellitusDisease cause
The present invention provides a novel compound represented by the formula (I) wherein each symbol is as defined in the specification, a salt thereof and a prodrug thereof having a superior GPR40 receptor function modulating action, which can be used as an insulin secretagogue, an agent for the prophylaxis or treatment of diabetes and the like. They unexpectedly show superior GPR40 receptor agonist activity, and also show superior properties as a pharmaceutical product, such as stability and the like. Thus, they can be safe and useful pharmaceutical agents for the prophylaxis or treatment of GPR40 receptor related diseases in mammals.
Owner:TAKEDA PHARMA CO LTD
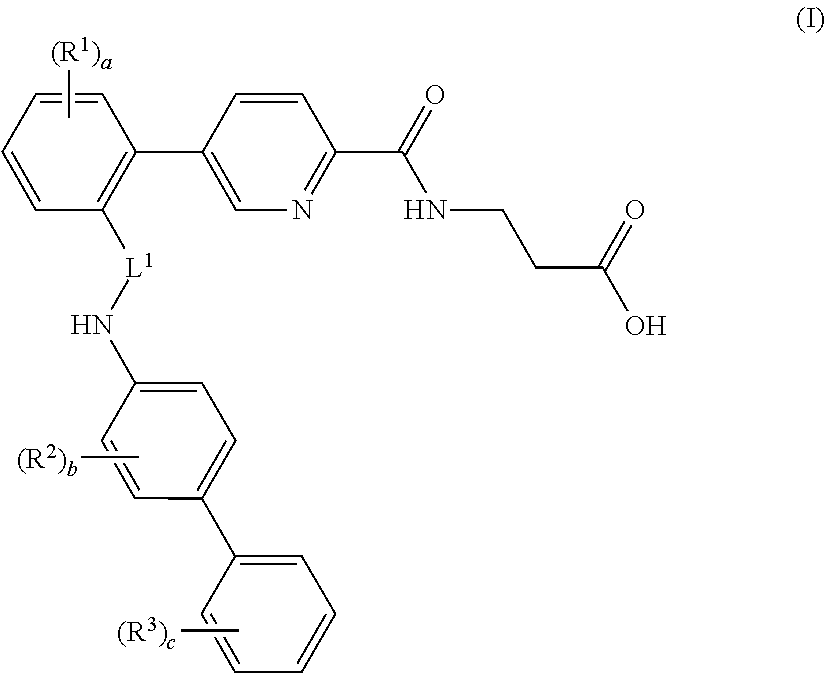
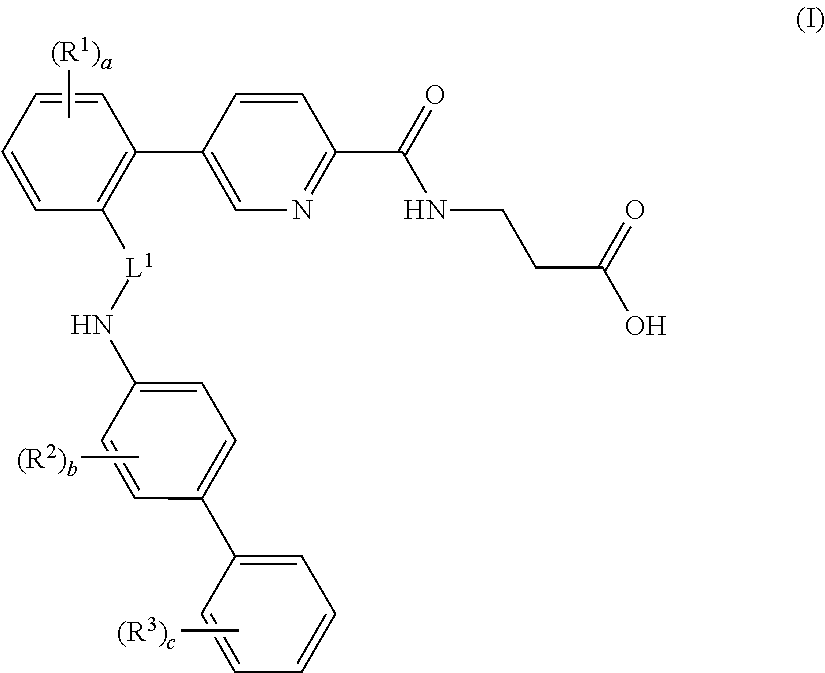
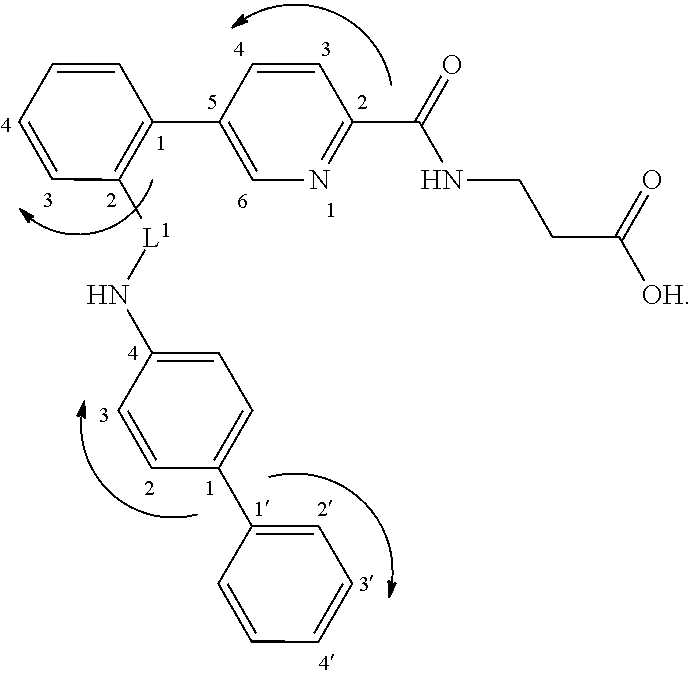
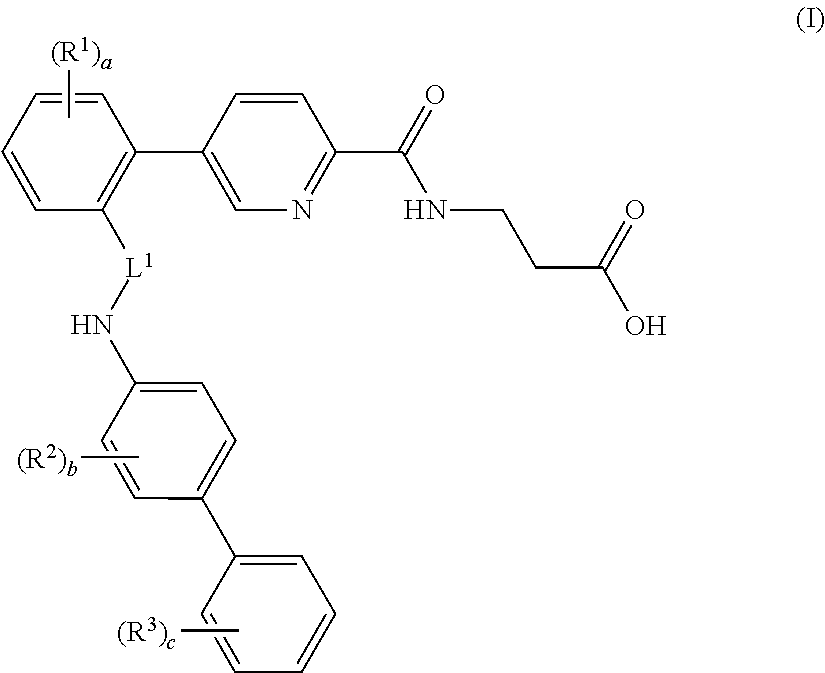
Picolinamido-propanoic acid derivatives useful as glucagon receptor antagonists
The present invention is directed to picolanmido-propanoic acid derivatives, pharmaceutical compositions containing them and their use in the treatment and / or prevention of disorders and conditions ameliorated by antagonizing one or more glucagon receptors, including for example metabolic diseases such as Type II diabetes mellitus and obesity.
Owner:JANSSEN PHARMA NV
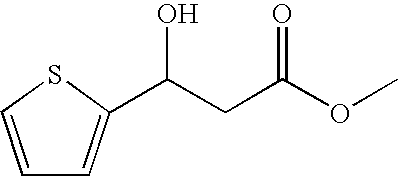
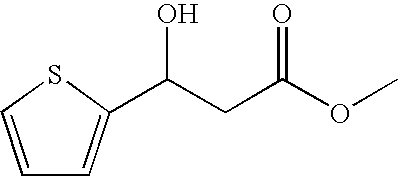
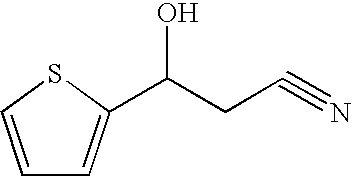
Process for preparing 3-heteroaryl-3-hydroxypropanoic acid derivatives
InactiveUS7553970B2High and high excess and high purityHigh yield and high excess and highOrganic chemistryMicroorganism based processes3-Hydroxypropionic acidAlcohol
The invention relates to a process for preparing enantiomer-enriched 3-heteroaryl-3-hydroxypropanoic acid derivatives and 3-heteroaryl-1-aminopropan-3-ols, and to their use.
Owner:LANXESS DEUTDCHLAND GMBH
3-(4-benzyloxyphenyl) propanoic acid derivatives
Owner:TAKEDA PHARMA CO LTD
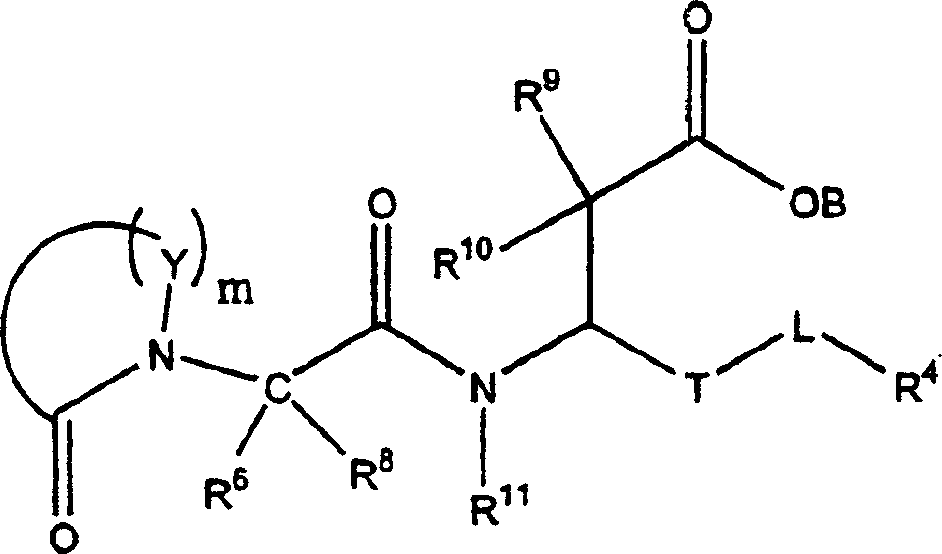
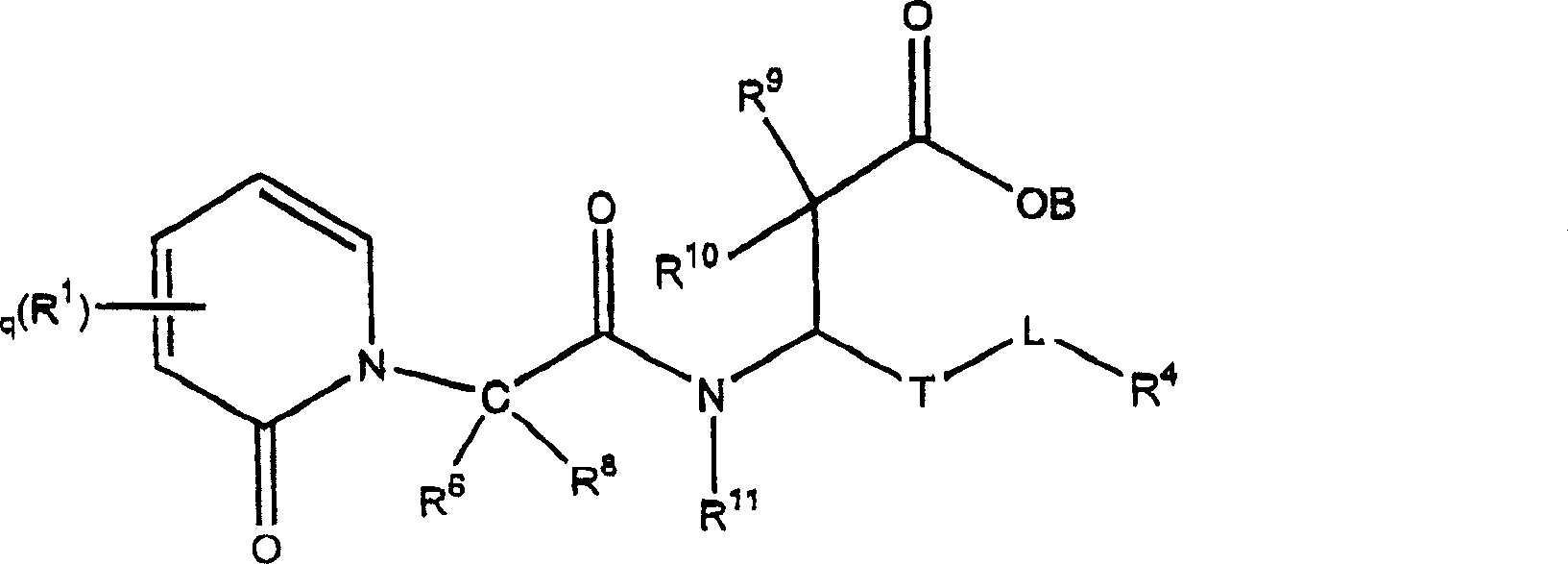
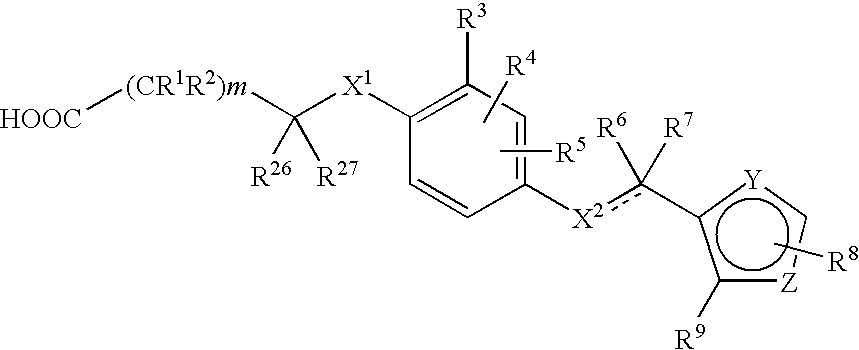
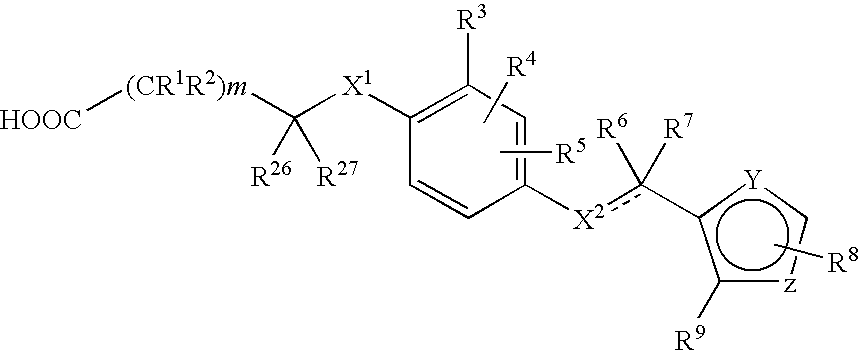
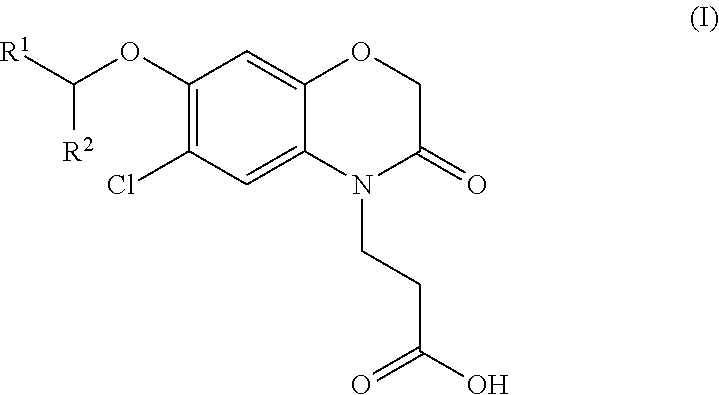
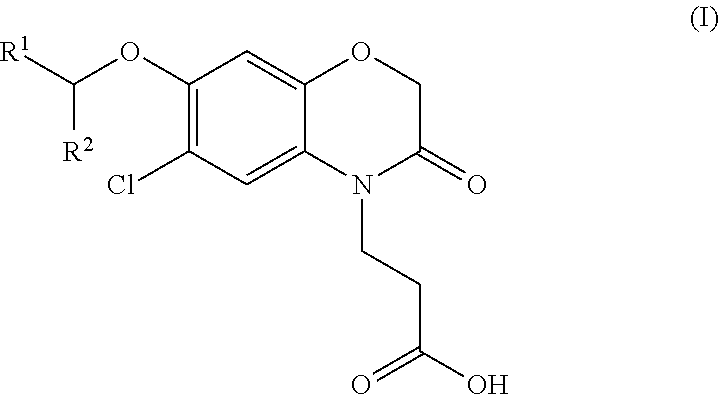
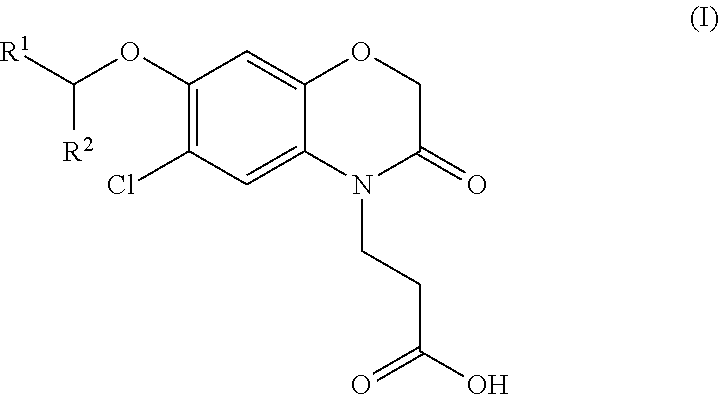
3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors
InactiveUS20160318884A1Antibacterial agentsOrganic active ingredientsAIDS dementia complexNeonatal respiratory distress syndrome
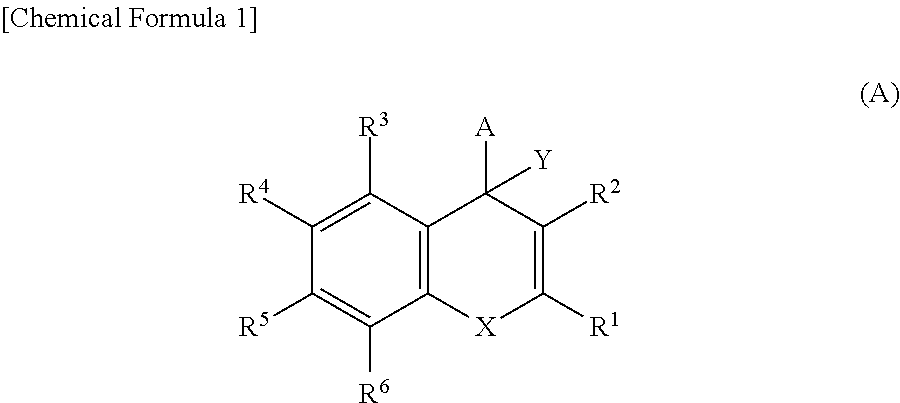
A compound of formula (I) or a salt thereof are provided:wherein R1, X and R3 are defined in the specification, useful in the treatment of disorders mediated by KMO such as acute pancreatitis, chronic kidney disease, other conditions associated with systemic inflammatory response syndrome (SIRS), Huntington's disease, Alzheimer's disease, spinocerebellar ataxias, Parkinson's disease, AIDS-dementia complex, amylotrophic lateral sclerosis (ALS), depression, schizophrenia, sepsis, cardiovascular shock, severe trauma, acute lung injury, acute respiratory distress syndrome, acute cholecystitis, severe burns, pneumonia, extensive surgical procedures, ischemic bowel, severe acute hepatic disease, severe acute hepatic encephalopathy or acute renal failure.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
3-(6-chloro-3-oxo-3,4-dihydro-(2H)-1,4-benzoxazin-4-yl) propanoic acid derivatives and their use as KMO inhibitors
Compounds of formula (I)wherein:R1 is heteroaryl optionally substituted by methyl, ethyl, halo or ═O; andR2 is H, methyl or ethyl.and salts thereof are KMO inhibitors and may be useful in the treatment of various disorders, for example acute pancreatitis, chronic kidney disease, other conditions associated with systemic inflammatory response syndrome (SIRS), Huntington's disease, Alzheimer's disease, spinocerebellar ataxias, Parkinson's disease, AIDS-dementia complex, HIV infection, amylotrophic lateral sclerosis (ALS), depression, schizophrenia, sepsis, cardiovascular shock, severe trauma, acute lung injury, acute respiratory distress syndrome, acute cholecystitis, severe burns, pneumonia, extensive surgical procedures, ischemic bowel disease, severe acute hepatic disease, severe acute hepatic encephalopathy or acute renal failure.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
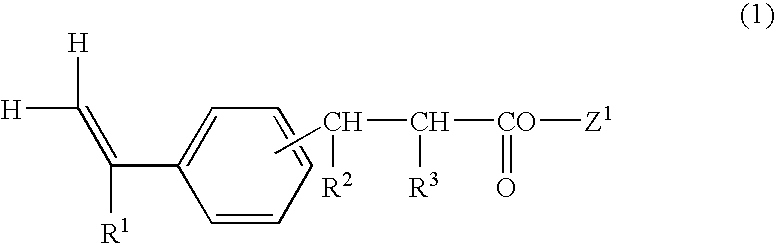
Vinylphenylpropionic acid derivatives, production process therefor, polymer thereof and radiation sensitive resin composition
InactiveUS6770780B1Reduce absorptionReduce the differenceOrganic compound preparationCarboxylic acid esters preparationResistAcetic acid
Vinylphenylpropionic acid derivatives; processes for producing the derivatives; polymers of the same; and radiosensitive resin compositions containing the polymers. The above polymers exhibit low radiation absorption and are useful as the resin component of radiosensitive resin compositions particularly suitable for chemically amplified resists. For example, t-butyl 4-vinylphenylpropionate is produced by (1) reacting t-butyl bromoacetate with tri(n-butyl)phosphine to obtain a quaternary phosphonium salt, (2) reacting this salt with a base to obtain a phosphorus ylide, (3) reacting this ylide with 2,4,6-tris(3',5'-di-t-butyl-4'-hydroxybenzyl)methyl-styrene to obtain a quaternary phosphonium salt, and (4) hydrolyzing this salt.
Owner:JSR CORPORATIOON
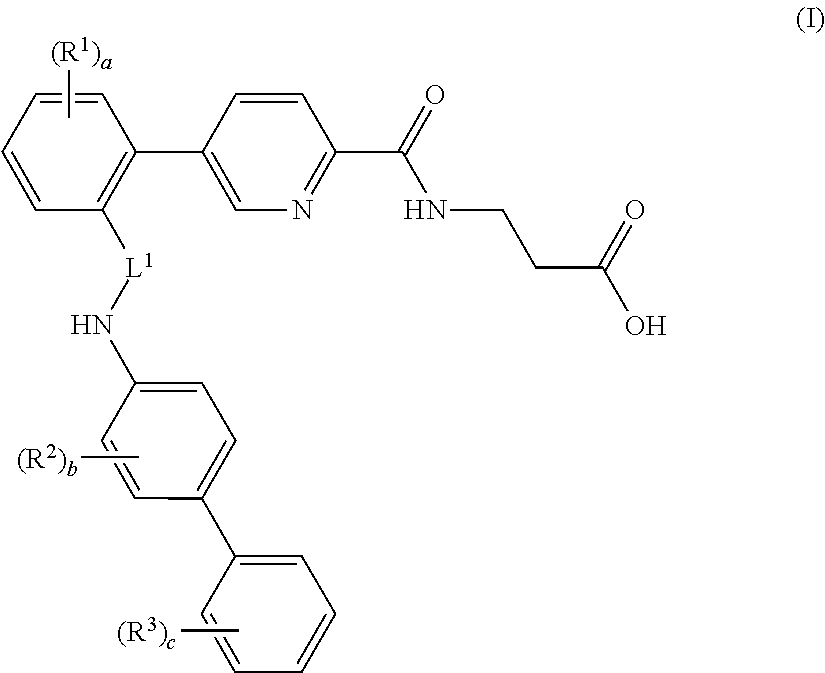
Picolinamido-propanoic acid derivatives useful as glucagon receptor antagonists
The present invention is directed to picolanmido-propanoic acid derivatives, pharmaceutical compositions containing them and their use in the treatment and / or prevention of disorders and conditions ameliorated by antagonizing one or more glucagon receptors, including for example metabolic diseases such as Type II diabetes mellitus and obesity.
Owner:JANSSEN PHARMA NV
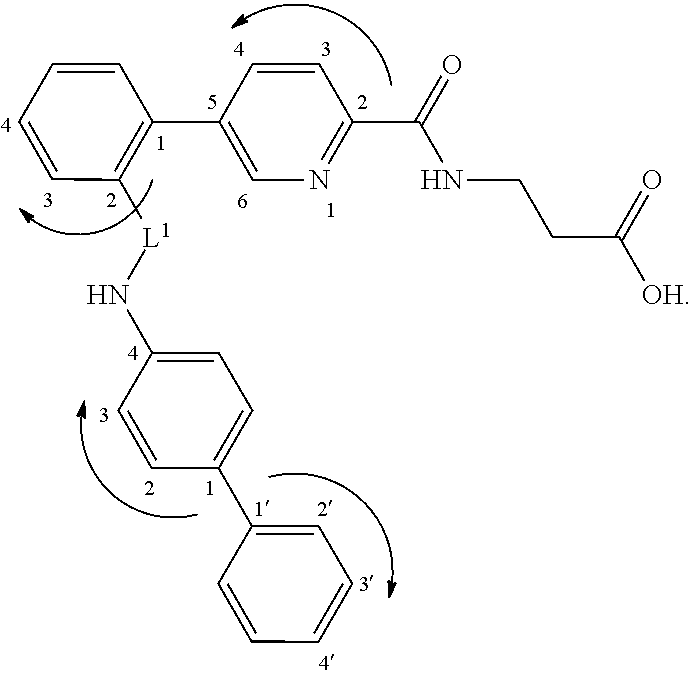
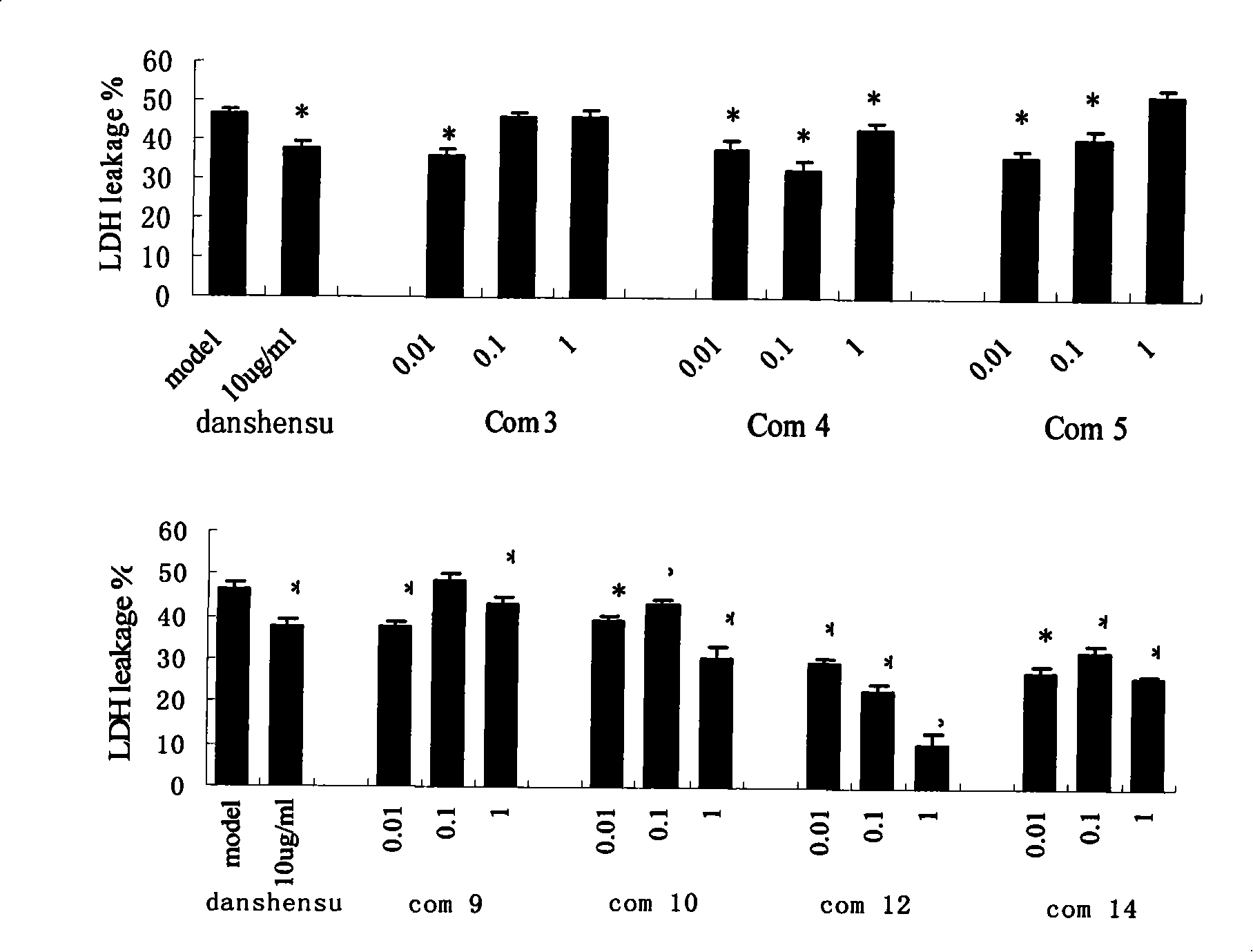
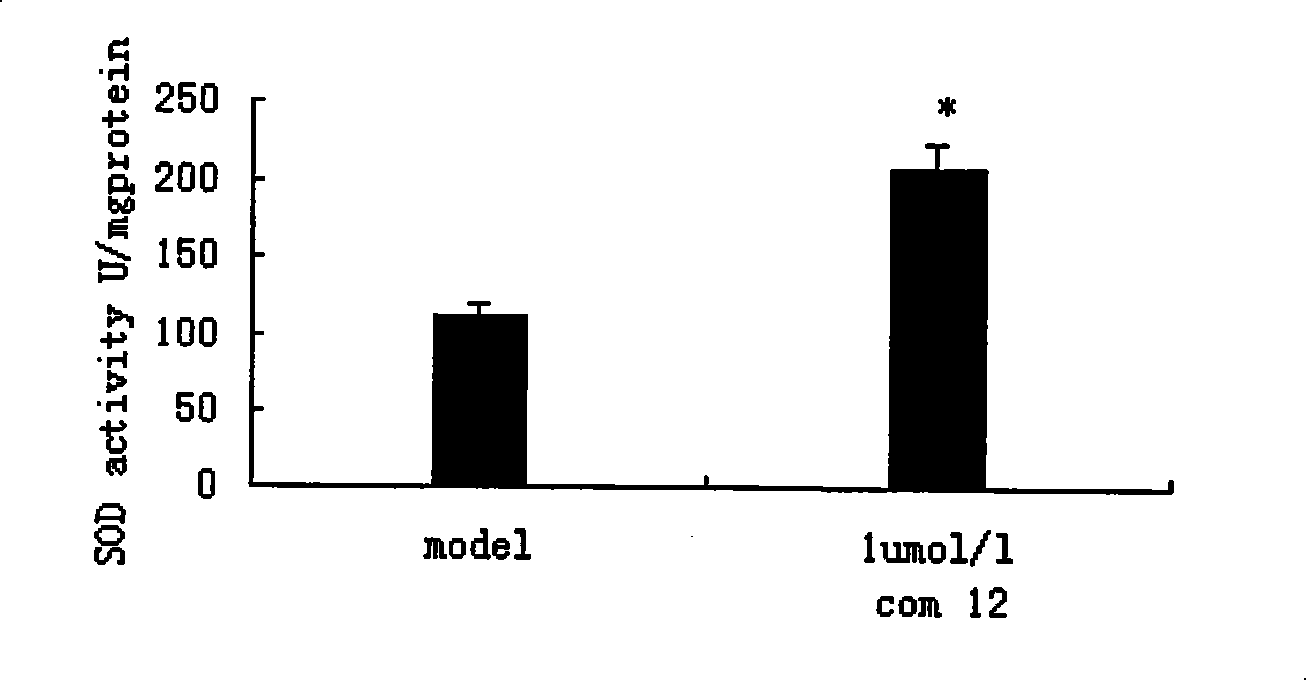
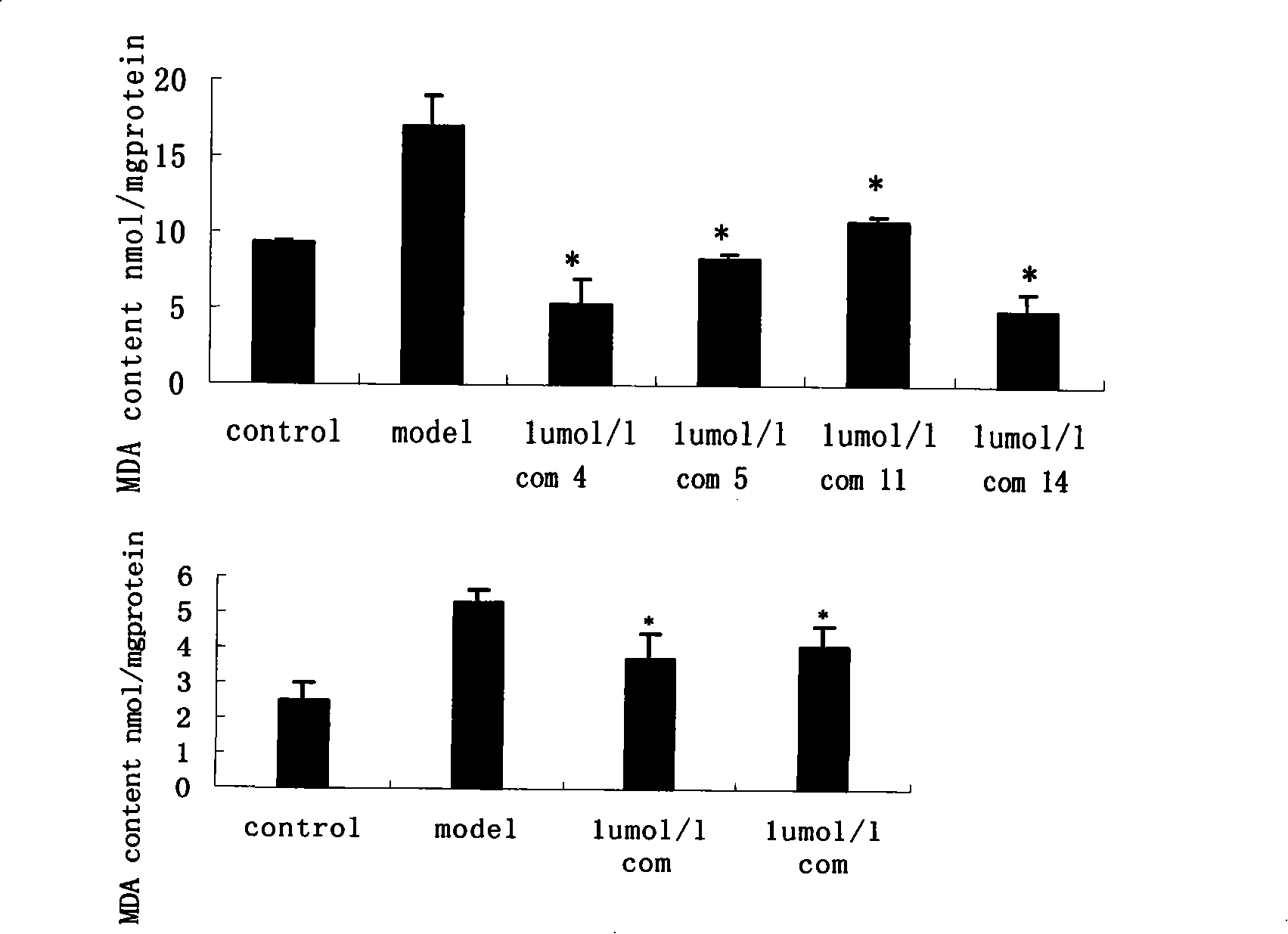
Propanoic acid derivatives and synthesis method and application thereof
ActiveCN101607904BProtectiveReduce the probability of leakageOrganic chemistryCardiovascular disorderAnti apoptotic genesCaspase
The invention belongs to the field of medicament synthesis, and relates to Danshensu derivatives with a structure in the following formula, a synthesis method thereof, and application thereof in pharmacy. The synthesis method adopts a chiral synthesis method to synthesize the Danshensu derivatives with stable structures in high yield, in particular derivatives of dextrorotatory Danshensu with high optical purity. Through experiments of animal models with hypoxic myocardial cells, results show that the Danshensu derivatives can reduce LDH leakage of the hypoxic myocardial cells, improve the activity of hyperoxide dismutase in the cells, inhibit generation of a product of lipid peroxidation, promote the expression of anti-apoptotic gene bcl-2 mRNA and inhibit the expression of pro-apoptosisgene bax mRNA and apoptotic gene caspase-3mRNA; and the results prove that the Danshensu derivatives have an effect of protecting hypoxic cardiac muscle and can be used for preparing medicaments for treating ischemic heart disease.
Owner:FUDAN UNIV
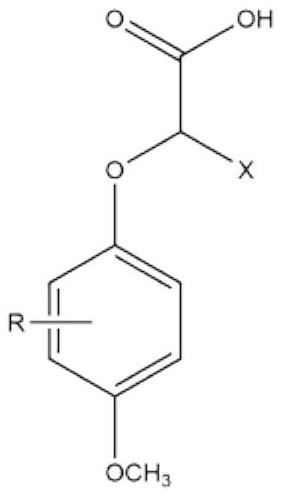
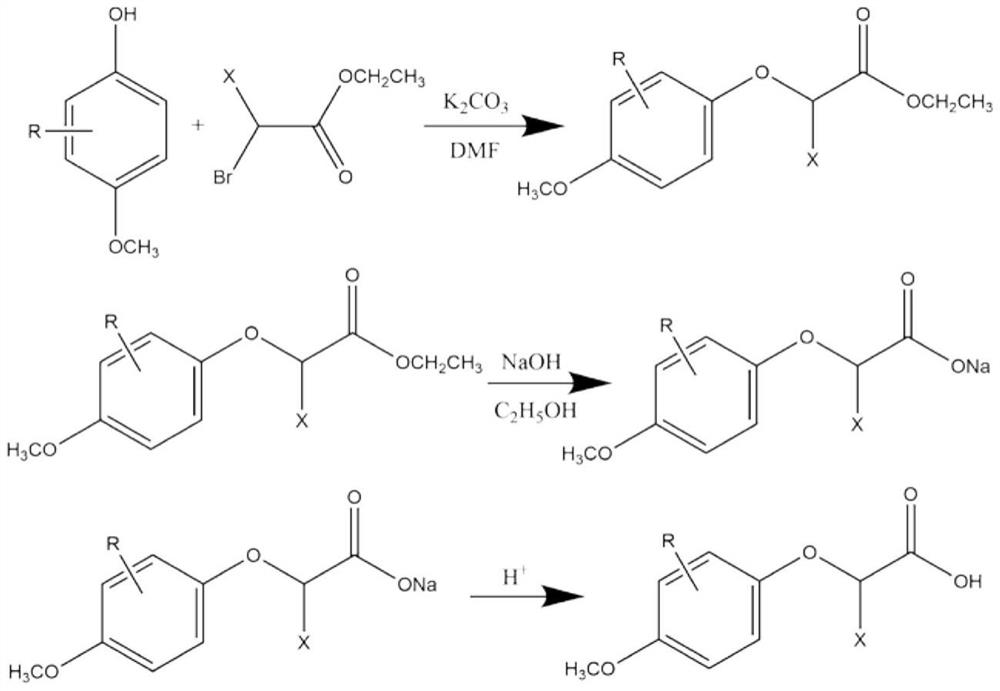
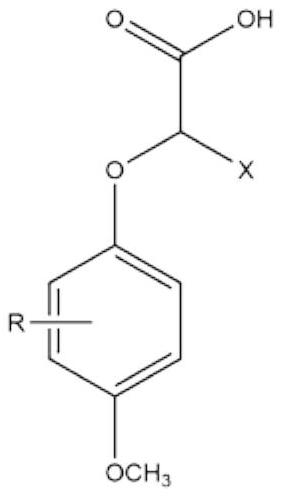
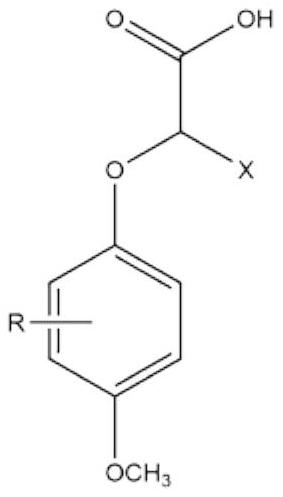
2-(4-methoxyphenoxy) propionic acid derivative with sweetness inhibiting effect and industrial production method of 2-(4-methoxyphenoxy) propionic acid derivative
ActiveCN112876354AWeak sour tasteImprove sweet and greasy tastePreparation from carboxylic acid esters/lactonesCarboxylic compound separation/purificationPropanoic acidSucrose
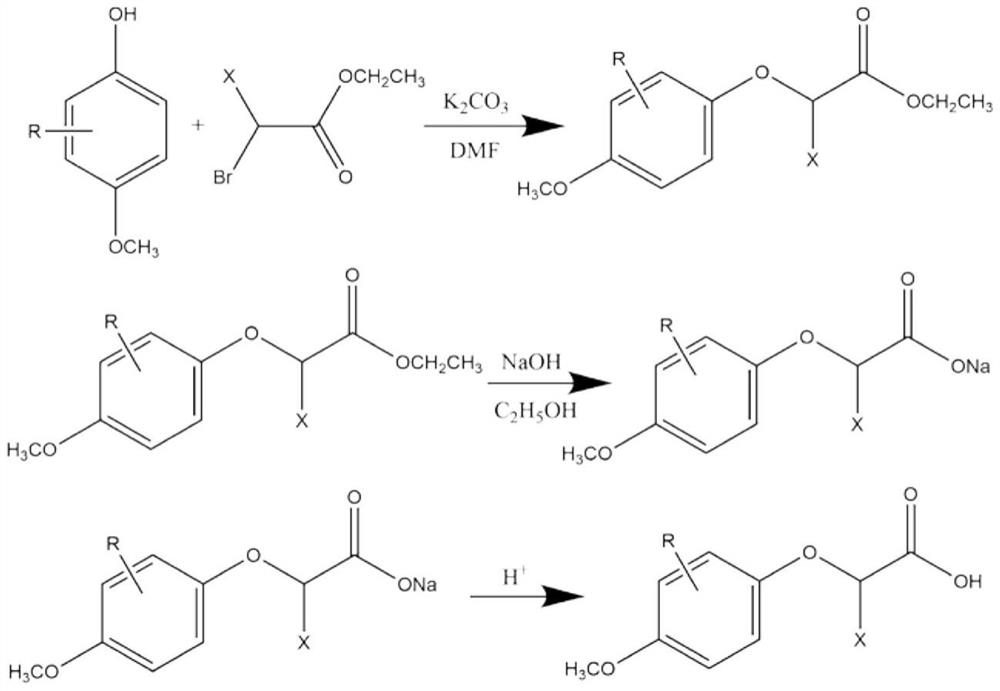
The invention discloses a 2-(4-methoxyphenoxy) propionic acid derivative with a sweetness inhibiting effect and an industrial production method of the 2-(4-methoxyphenoxy) propionic acid derivative. The structural general formula is shown in the specification, in the formula, R represents C1-C4 alkyl, C1-C4 alkoxy, nitryl or halogen atoms, and X represents hydrogen atoms or C1-C3 alkyl. The preparation method comprises the following steps: mixing p-methoxy substituted phenol, ethyl 2-bromoalkanoate and potassium carbonate, reacting in dimethylformamide, and filtering; adding water and an organic phase into the filtrate, carrying out liquid separation treatment, and evaporating to remove the organic phase to obtain an ester compound; and adding the obtained ester and sodium hydroxide into ethanol, carrying out reflux reaction, acidifying, extracting, recrystallizing and the like to obtain the 2-(4-methoxyphenoxy) propionic acid derivative. The derivative is a brand-new sweetness inhibiting compound and has a good sweetness inhibiting effect on cane sugar, the industrial method is mild in reaction condition, the temperature does not exceed 100 DEG C, separation and purification are simple, and the product purity is high.
Owner:SOUTH CHINA UNIV OF TECH
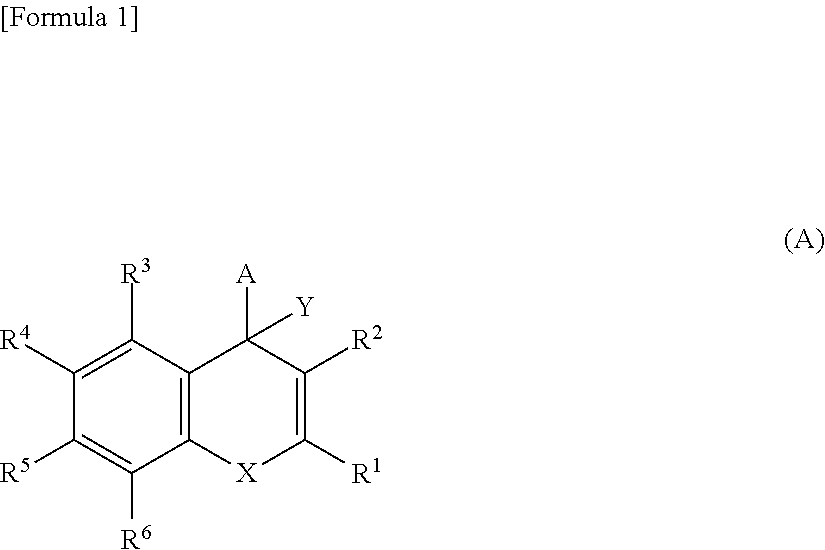
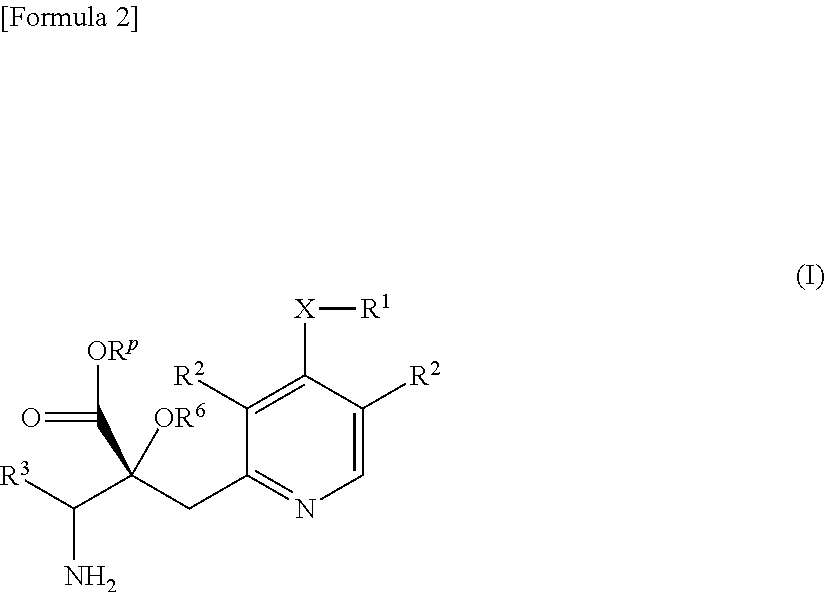
Pyridine derivative
ActiveUS20170190715A1Improve the level ofReduce productionOrganic chemistryUrinary disorderPropanoic acidPlacental leucine aminopeptidase
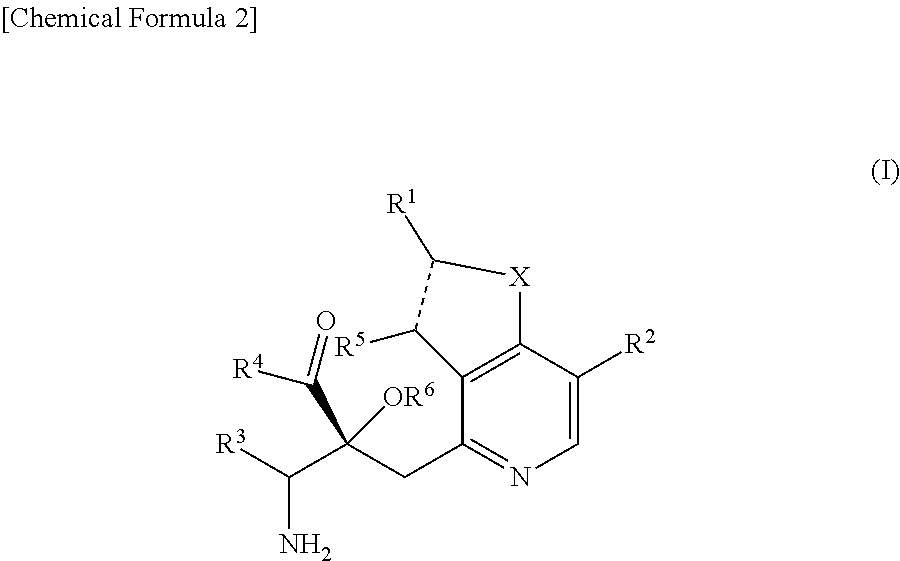
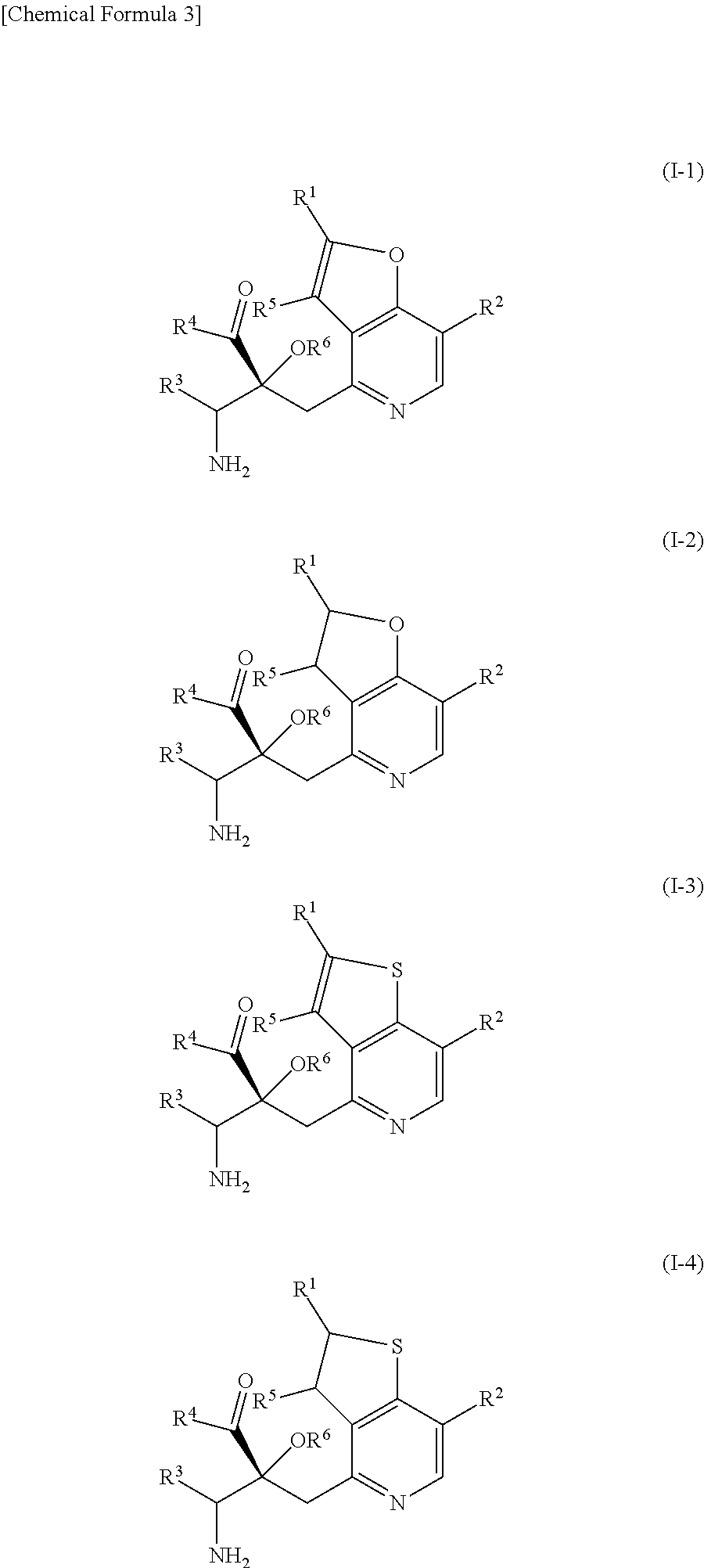
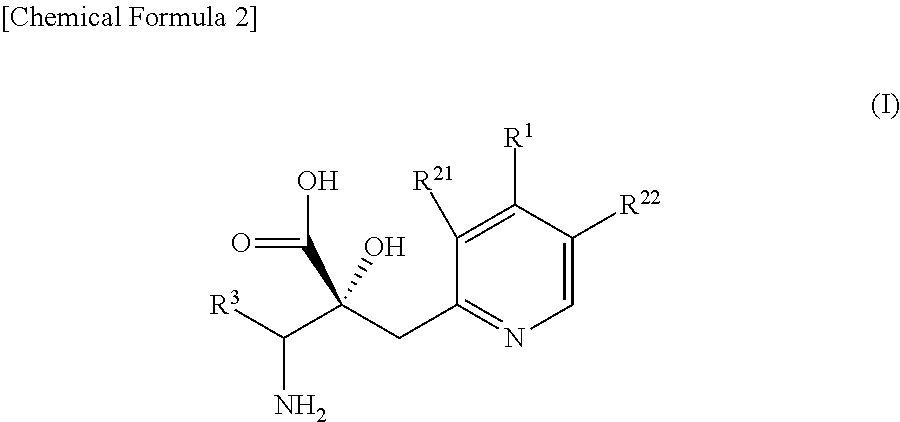
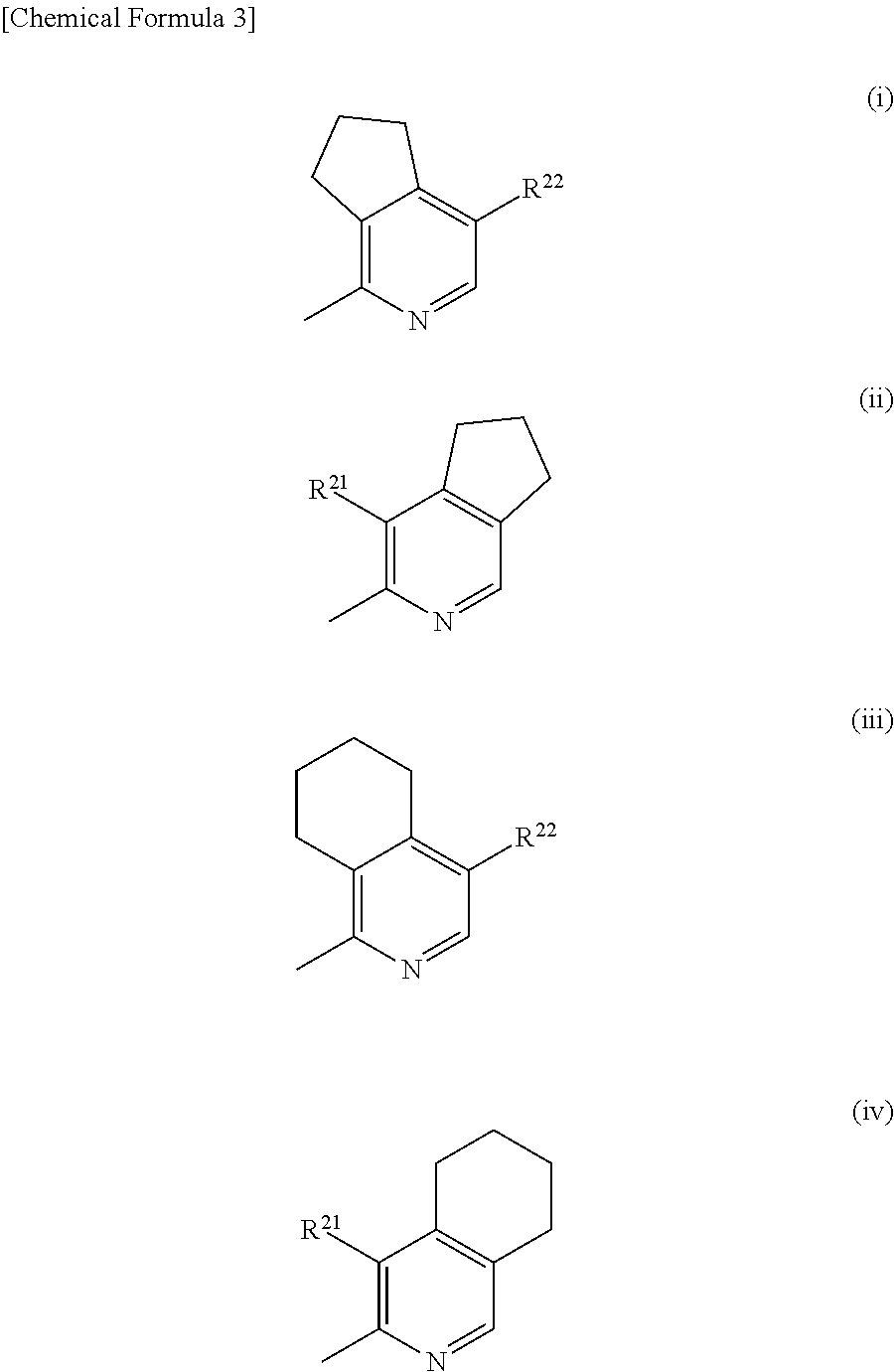
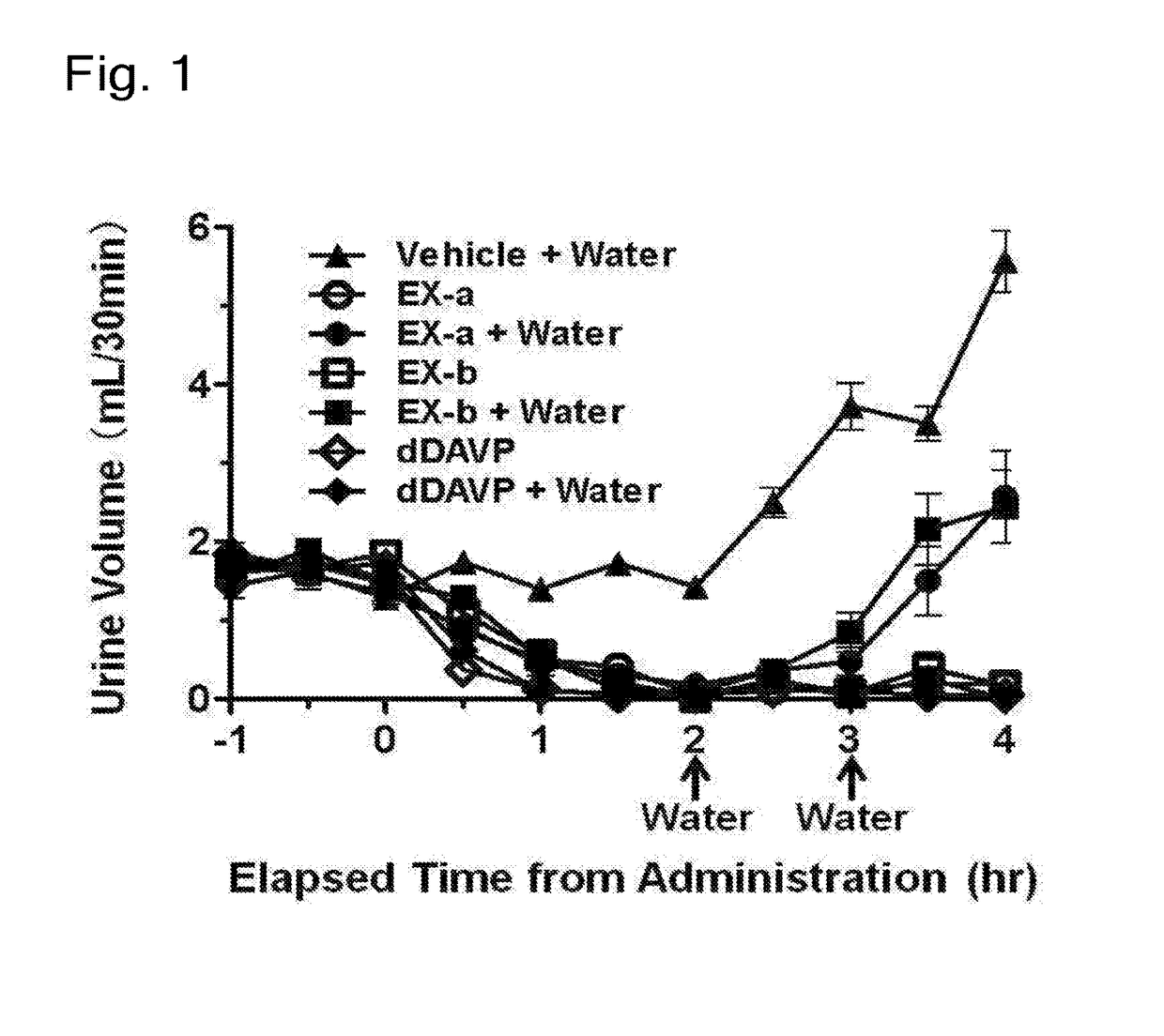
The problem to be solved by the present invention is to provide a compound suitable for a pharmaceutical composition, specifically a pharmaceutically composition for treating nocturia.The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, would maintain and / or increase an endogenous AVP level to enhance the antidiuretic effect, which would contribute to a decreased number of nocturnal voids, and have extensively studied compounds which inhibit P-LAP.As a result, the inventors have found that (2R)-3-amino-2-{[4-(substituted pyridine)-2-yl]methyl}-2-hydroxy-propanoic acid derivatives have excellent P-LAP inhibitory activity. The inventors have evaluated antidiuretic effects in water-loaded rats and have found that the compounds increase endogenous AVP levels by inhibiting P-LAP and consequently reduce urine production. The present invention therefore provides compounds expected to be used as an agent for treating nocturia based on P-LAP inhibition.
Owner:ASTELLAS PHARMA INC +1
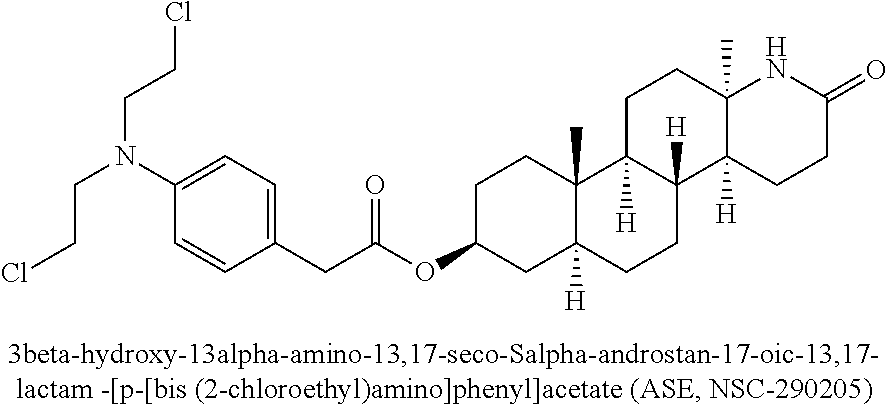
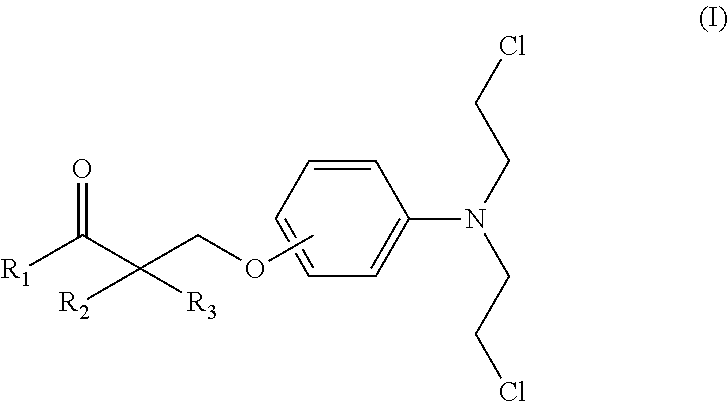
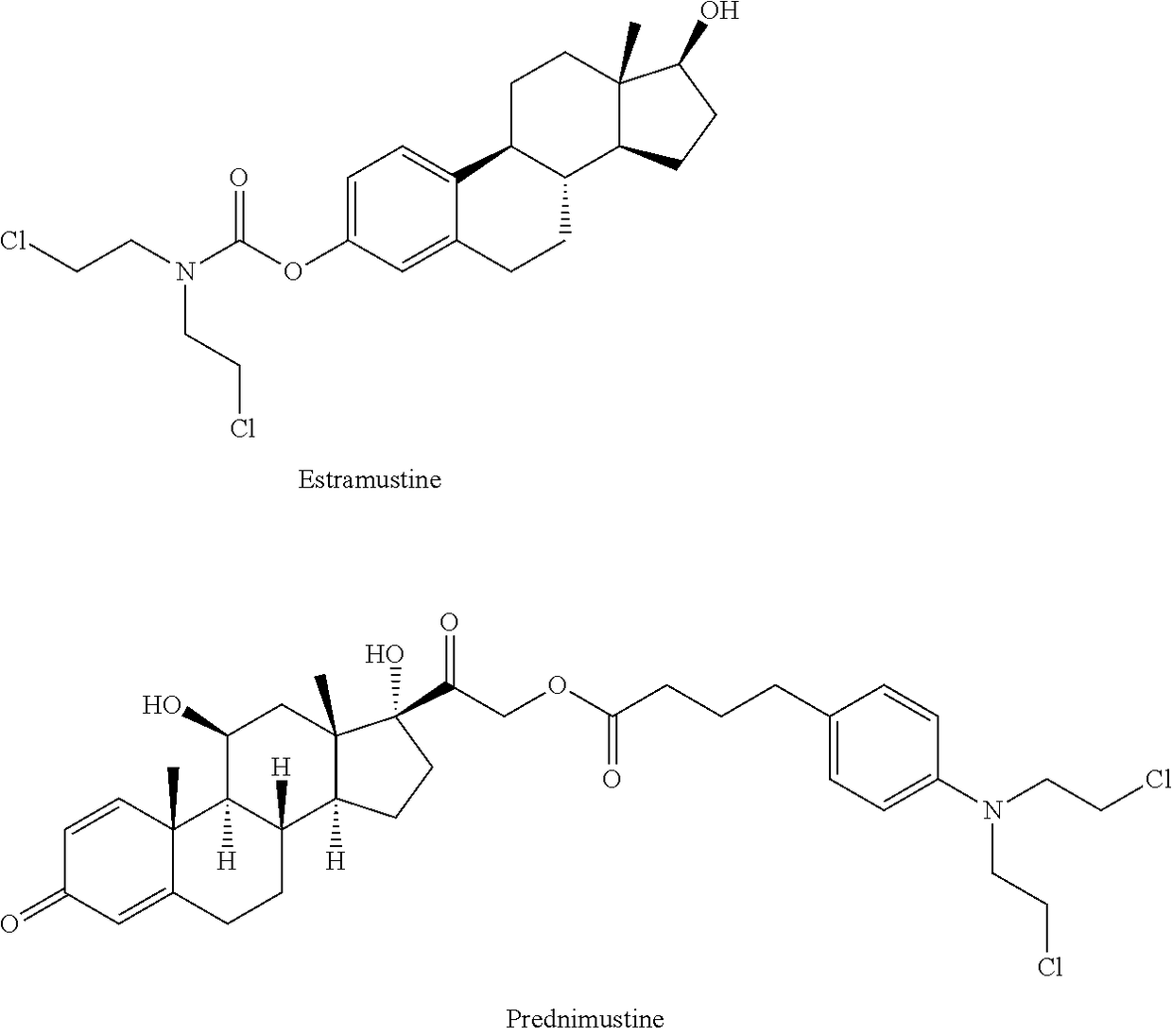
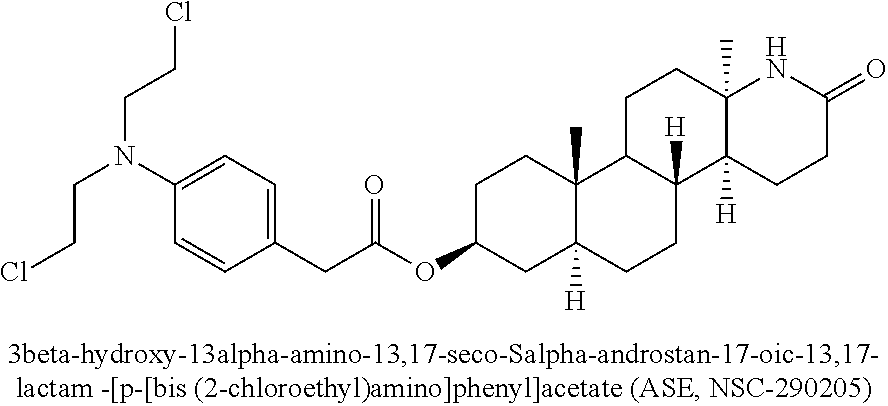
Esters of steroidal lactam and bis(2-chloroethyl) aminophenoxy propanoic acid derivatives
ActiveUS20180194800A1Improve anti-tumor activityLow acute toxicityOrganic active ingredientsSteroidsPropynoic acidPropanoic Acid Derivatives
Novel homo-aza-steroidal esters with alkylating bis(2-chloroethyl)aminophenoxy propanoic acid and substituted derivatives, processes for their preparation, pharmaceutical compositions containing them and use thereof in the treatment of cancer.
Owner:GALENICA SA +1
Process for production of optically active-3-amino-2-hydroxypropionic cyclopropylamide derivatives and salts thereof
InactiveUS7834190B2Easy to getOrganic compound preparationCarboxylic acid esters preparationOxazocinesAcid derivative
An objective of the present application is to provide an industrially practicable method for producing an optically-active 3-amino-2-hydroxypropionic cyclopropylamide derivative or salt thereof from an inexpensive easily-available starting material. The derivative or salt thereof is useful as an intermediate for a medicine. It is also intended by the present application to provide a useful intermediate of the derivative. The objective is attained by the following method. First, an easily-available 2-halo-3-oxopropionic acid derivative is asymmetrically reduced, and then epoxidated to produce an optically-active epoxycarboxylic acid derivative. Next, the derivative is converted into an optically-active epoxyamide derivative by reaction with cyclopropylamine, and then reacted with a nitrile to obtain an optically-active oxazolinamide derivative. Subsequently, selective acid solvolysis of the oxazoline skeleton gives the optically-active 3-amino-2-hydroxypropionic cyclopropylamide derivative or salt thereof.
Owner:KANEKA CORP
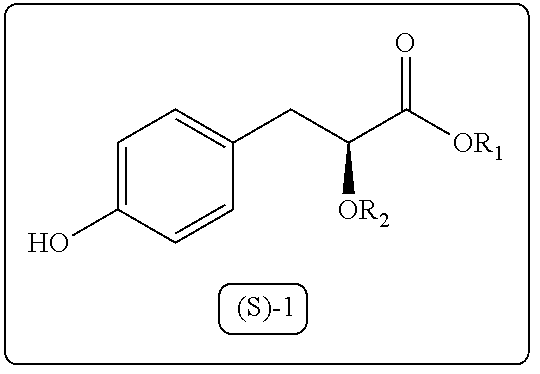
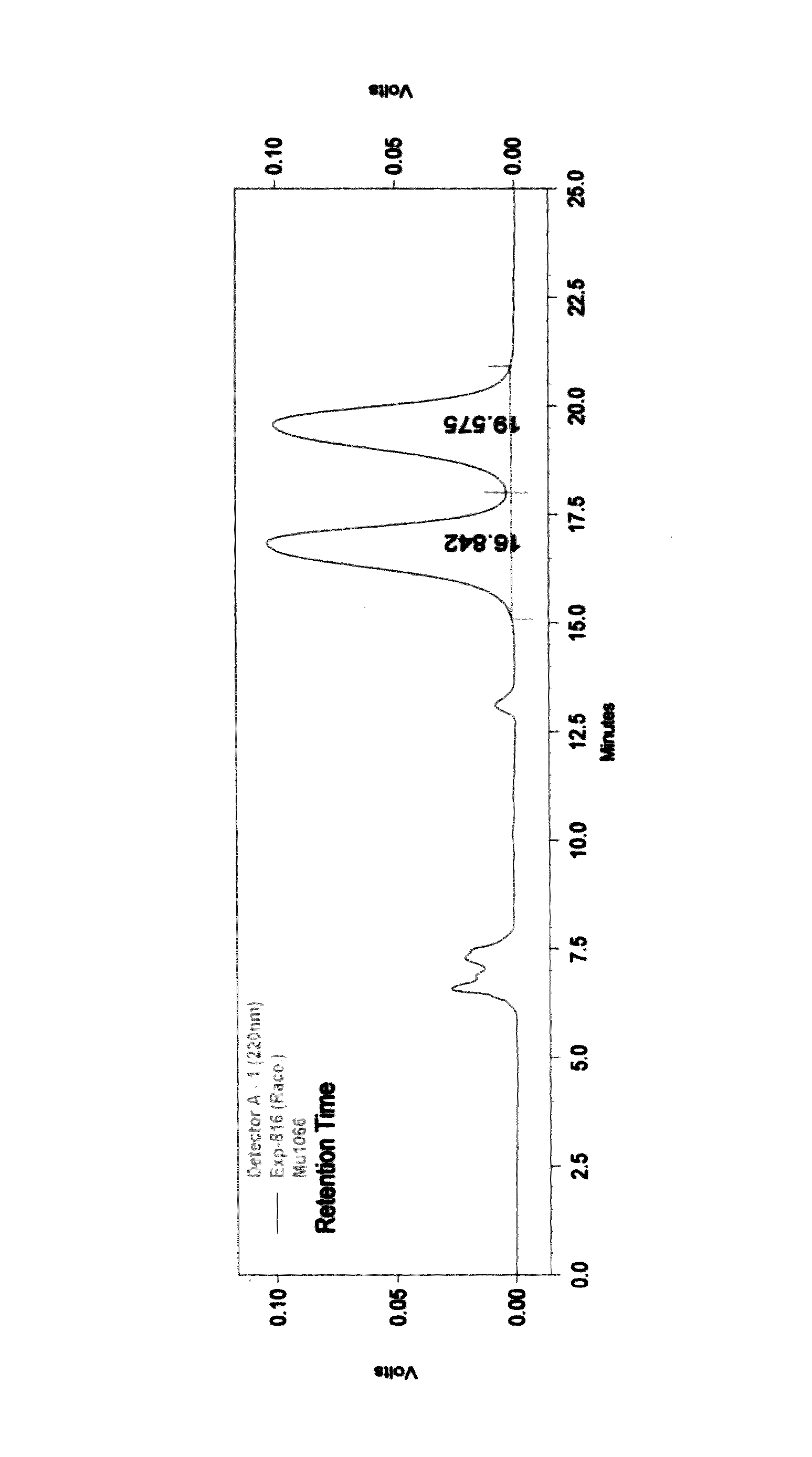
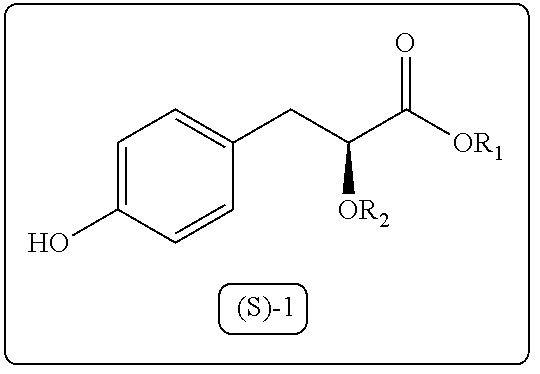
Process for the preparation of 3-aryl-2-hydroxy propanoic acid compounds
ActiveUS9550719B2High enantiomeric purityHigh yieldPreparation from carboxylic acid halidesOrganic compound preparationArylPropanoic acid
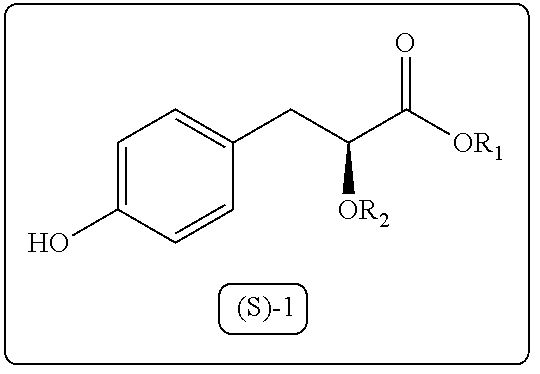
The present disclosure provides a process for synthesis of 3-aryl-2-hydroxy propanoic acid derivatives of formula (S)-1. wherein R1 represents H or (C1-C5) alkyl groups and R2 represents (C1-C5) alkyl groups.
Owner:COUNCIL OF SCI & IND RES
Propanoic acid derivatives that inhibit binding of integrins to their receptors
The present invention relates to methods of inhibiting the binding of α4β1 integrin to its receptors such as VCAM-1 (vascular cell adhesion molecule-1) and fibronectin; compounds that inhibit such binding; pharmaceutically active compositions containing such compounds; and Use of such compounds in the above aspects or in preparations for controlling or preventing disease states in which α4β1 is involved.
Owner:ENCYSIVE PHARM INC NEW YORK NEW
3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
Process for the purification of 2-phenyl-2-methyl-propanoic acid derivatives
ActiveUS9334223B2Preparation from carboxylic acid saltsOrganic compound preparationPhenyl groupFexofenadine Hydrochloride
Process for the preparation of 2-phenyl-2-methyl propanoic acid derivatives, useful as intermediates in the preparation of fexofenadine hydrochloride.
Owner:DIPHARMA FRANCIS
Process for producing 3-amino-2-hydroxypropionic acid derivatives
InactiveUS7057066B2High yieldQuality improvementCarbamic acid derivatives preparationOrganic compound preparationLeaving group3-Aminopropionic Acid
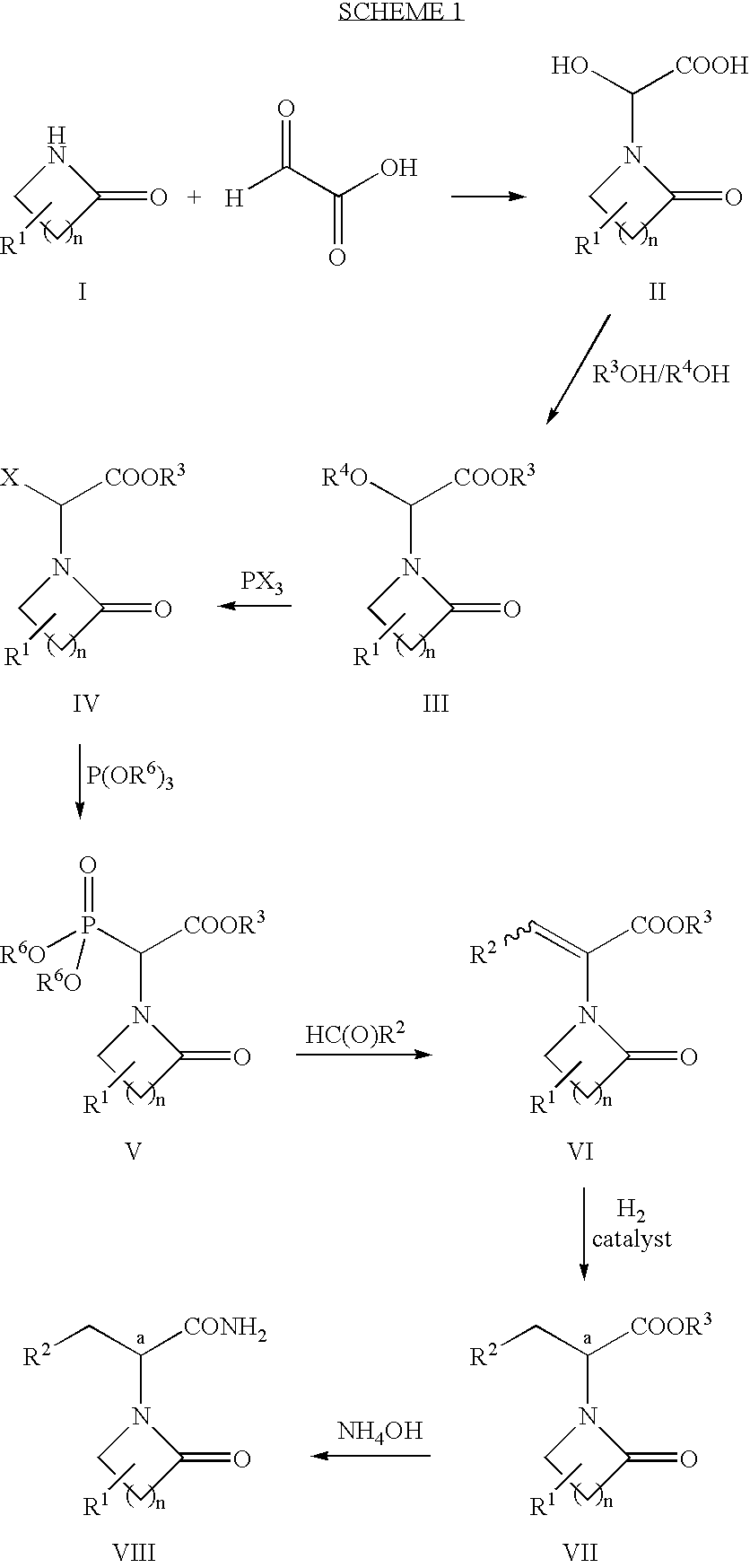
The present invention provides a process for preparing 3-amino-2-hydroxypropionic acid derivatives (1) which does not use dangerous reagents, is economically advantageous, and is suitable for an industrial production, which process comprises:treating N-protected-3-amino-2-hydroxypropionic acid derivatives (2) having a steric configuration at 2-position carbon reverse to that of derivatives (1) with a leaving group-introducing agent to convert into N-protected-3-aminopropionic acid derivatives (3),then treating the derivatives with a basic substance to convert into substituted-3-amino-2-hydroxypropionic acid derivatives (4) having an inverted steric configuration at 2-position carbon,and then converting the derivatives into 3-amino-2-hydroxypropionic acid derivatives (1).
Owner:KANEKA CORP
Process for the purification of 2-phenyl-2-methyl-propanoic acid derivatives
ActiveUS20150099899A1Preparation from carboxylic acid saltsOrganic compound preparationPhenyl groupFexofenadine Hydrochloride
Process for the preparation of 2-phenyl-2-methyl propanoic acid derivatives, useful as intermediates in the preparation of fexofenadine hydrochloride.
Owner:DIPHARMA FRANCIS
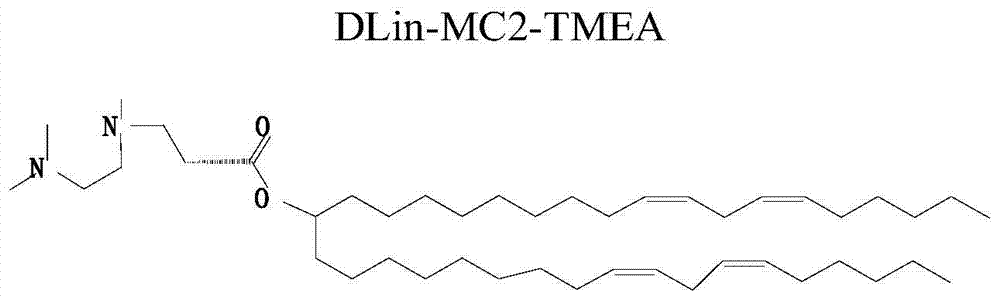
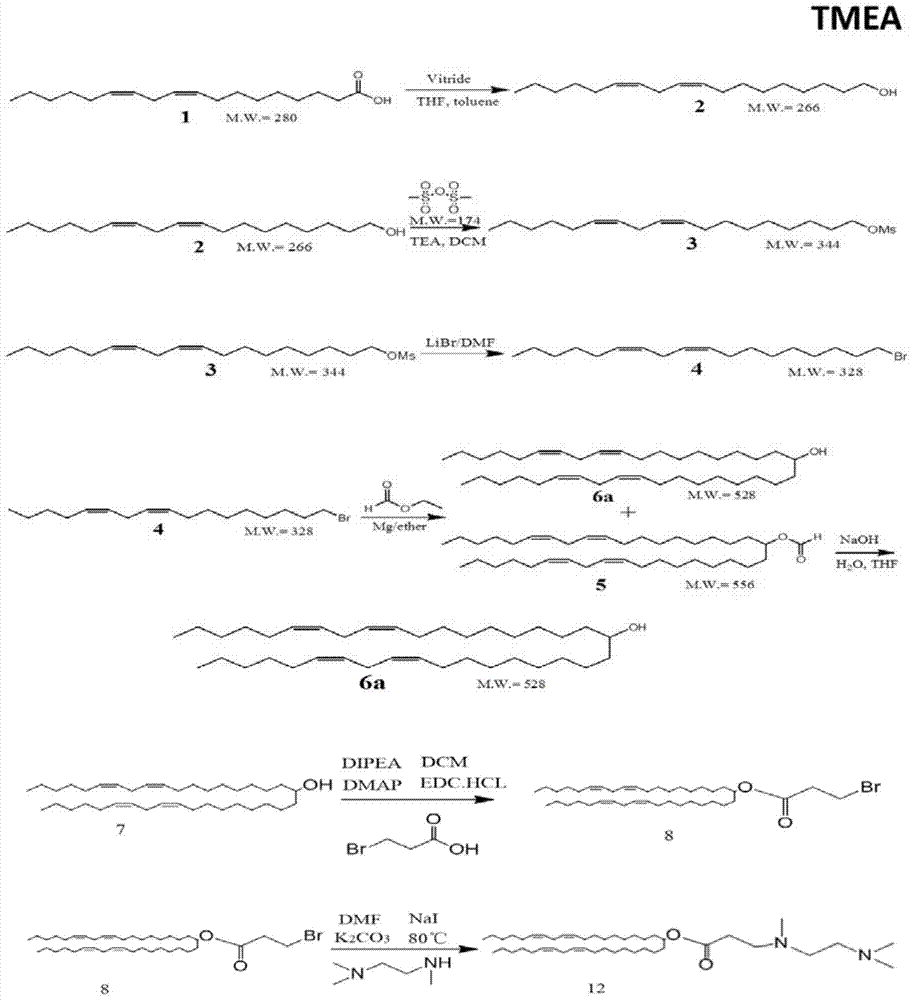
Amphiphilic derivatives of 3-((2-(dimethylamino)ethyl)(methyl)amino)propionic acid and uses thereof
ActiveCN105085292BIncrease loading capacityImprove in vivo stabilityOrganic compound preparationGenetic material ingredientsFluorescenceCytotoxicity
The present invention relates to a kind of amphiphilic derivative of 3-((2-(dimethylamino) ethyl) (methyl) amino) propionic acid and its use; Prepared liposome; the use is the use of liposome as a drug carrier delivery system. The liposome prepared from the amphiphilic compound of the present invention can carry more positive charges at acidic pH, can better compound gene drug siRNA, and form a complex with smaller particle size and uniform particle size distribution. At the same time, it is electrically neutral under the pH7.4 environment, which increases the stability of the lipoplex in vivo and reduces the cytotoxicity caused by excessive positive charges. The liposome provided by the invention can specifically inhibit gene expression in human non-small cell lung cancer H1299‑Pgl3 cells in vitro, and can specifically transfer fluorescent gene drugs into normal mouse liver cells in vivo.
Owner:SHANGHAI JIAOTONG UNIV
Esters of steroidal lactam and bis(2-chloroethyl) aminophenoxy propanoic acid derivatives
ActiveUS10208083B2Improve anti-tumor activityLow acute toxicityOrganic active ingredientsSteroidsPropanoic acidPropanoic Acid Derivatives
Owner:GALENICA SA +1
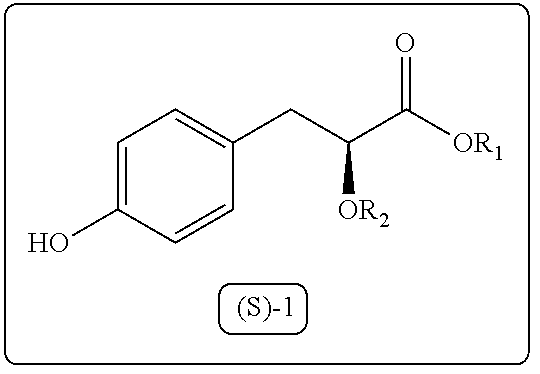
A process for the preparation of 3-aryl-2-hydroxy propanoic acid compounds
ActiveUS20160102042A1High enantiomeric purityHigh yieldPreparation from carboxylic acid halidesOrganic compound preparationArylPropanoic acid
The present disclosure provides a process for synthesis of 3-aryl-2-hydroxy propanoic acid derivatives of formula (S)-1. wherein R1 represents H or (C1-C5) alkyl groups and R2 represents (C1-C5) alkyl groups.
Owner:COUNCIL OF SCI & IND RES
Highly enantiomerically pure lactam-substituted propanoic acid derivatives and methods of making and using same
InactiveUS6897323B2Carbamic acid derivatives preparationCarboxylic acid nitrile preparationPropanoic acidAsymmetric hydrogenation
The present invention relates to highly enantiomerically pure lactam-substituted propanoic acid derivatives and methods of making and using therefor. The invention involves a multi-step synthesis to produce the lactam compounds. In one step of the reaction sequence, asymmetric hydrogenation of a lactam-enamide was performed to produce an intermediate that can ultimately be converted to a series of pharmaceutical compounds. The invention also contemplates the in situ synthesis of an intermediate of the multi-step synthesis, which provides economic advantages to the overall synthesis of the lactam compounds.
Owner:JOHNSON MATTHEY PLC
Propanoic acid derivatives that inhibit binding of integrins to their receptors
A method for the inhibition of the binding of alpha<4>beta<1> integrin to its receptors, for example VCAM-1 (vascular cell adhesion molecule-1) and fibronectin; compounds that inhibit this binding; pharmaceutically active compositions comprising such compounds; and the use of such compounds either as above, or in formulations for the control or prevention of diseases states in which alpha<4>beta<1> is involved.
Owner:ENCYSIVE PHARM INC NEW YORK NEW
Process for producing 3-amino-2-hydroxypropionic acid derivatives
InactiveUS20060135784A1Quality improvementReduce yieldOrganic compound preparationAmino-carboxyl compound preparationBiochemical engineeringAcid derivative
The present invention provides a process for preparing 3-amino-2-hydroxypropionic acid derivatives (1) which does not use dangerous reagents, is economically advantageous, and is suitable for an industrial production, which process comprises: treating N-protected-3-amino-2-hydroxypropionic acid derivatives (2) having a steric configuration at 2-position carbon reverse to that of derivatives (1) with a leaving group-introducing agent to convert into N-protected-3-aminopropionic acid derivatives (3), then treating the derivatives with a basic substance to convert into substituted-3-amino-2-hydroxypropionic acid derivatives (4) having an inverted steric configuration at 2-position carbon, and then converting the derivatives into 3-amino-2-hydroxypropionic acid derivatives (1).
Owner:KANEKA CORP
Bicyclic pyridine compound
ActiveUS10023583B2Improve the level ofReduce productionOrganic active ingredientsOrganic chemistryMedicinePlacental leucine aminopeptidase
The problem to be solved by the present invention is to provide a compound suitable for a pharmaceutical composition, specifically a pharmaceutically composition for treating nocturia.The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, would maintain and / or increase an endogenous AVP level to enhance the antidiuretic effect, which would contribute to a decreased number of nocturnal voids, and have extensively studied compounds which inhibit P-LAP. As a result, the inventors have found that (2R)-3-amino-2-(bi-cyclic pyridylmethyl)-2-hydroxy-propanoic acid derivatives have excellent P-LAP inhibitory activity. The inventors have evaluated antidiuretic effects in water-loaded rats and have found that the compounds increase endogenous AVP levels by inhibiting P-LAP and consequently reduce urine production. The present invention therefore provides compounds expected to be used as an agent for treating nocturia based on P-LAP inhibition.
Owner:ASTELLAS PHARMA INC +1
2-(4-methoxyphenoxy)propionic acid derivatives with sweet taste inhibitory effect and industrial production method thereof
ActiveCN112876354BWeak sour tasteImprove sweet and greasy tastePreparation from carboxylic acid esters/lactonesCarboxylic compound separation/purificationPropanoic acidSucrose
Owner:SOUTH CHINA UNIV OF TECH
Pyridine derivatives
ActiveUS20170362209A1Reduce urine productionIncrease endogenous AVP levelOrganic chemistryBlood disorderBacteriuriaMedicine
The problem to be solved by the present invention is to provide a compound suitable for a pharmaceutical composition, specifically an agent for treating nocturia.The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, would maintain and / or increase an endogenous AVP level to enhance the antidiuretic effect, which would contribute to a decreased number of nocturnal voids, and have extensively studied compounds which inhibit P-LAP. As a result, the inventors have found that (2R)-3-amino-2-(pyridylmethyl)-2-hydroxy-propanoic acid derivatives have excellent P-LAP inhibitory activity. The inventors have evaluated antidiuretic effects in water-loaded rats and have found that the compounds increase endogenous AVP levels by inhibiting P-LAP and consequently reduce urine production. The present invention therefore provides compounds expected to be used as an agent for treating nocturia based on P-LAP inhibition.
Owner:ASTELLAS PHARMA INC +1
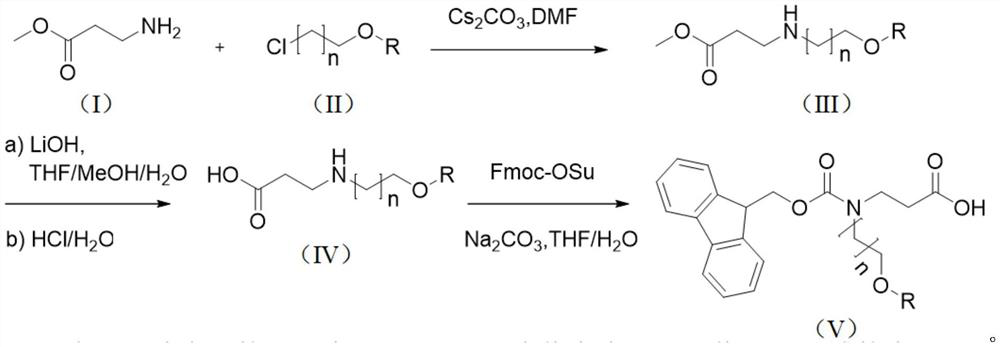
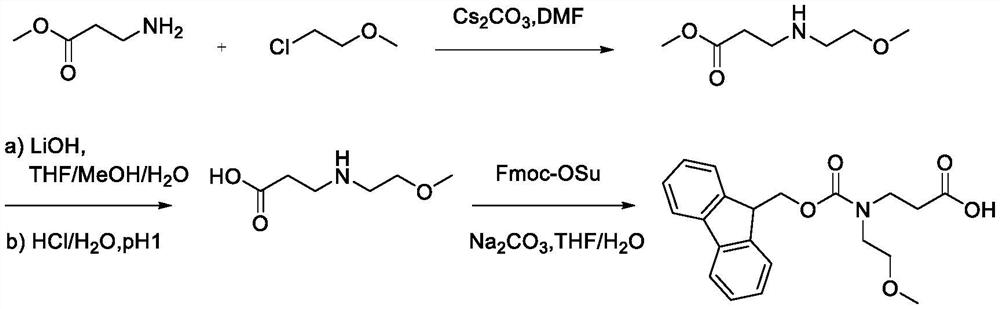
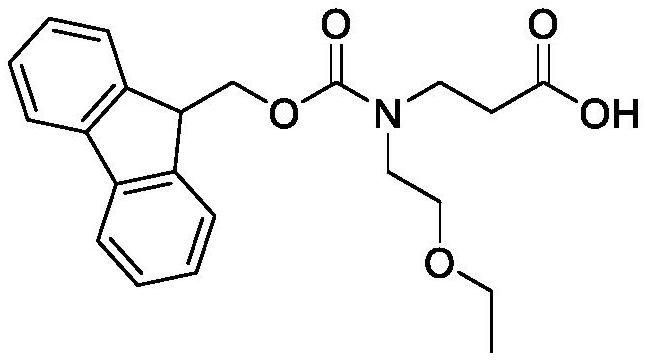
Synthesis method of N-Fmoc-3-aminopropionic acid derivative
InactiveCN113999141AHigh yieldThe synthesis method is simpleCarbamic acid derivatives preparationOrganic compound preparationImidePropanoic acid
The invention relates to a synthesis method of an N-Fmoc-3-aminopropionic acid derivative. The method comprises the following steps: (1) reacting a compound (I) with a compound (II) in the presence of a solvent and alkali to obtain a compound (III); (2) carrying out a hydrolysis reaction on the a compound (III) to obtain a compound (IV); and (3) reacting the compound (IV) with fluorenylmethoxycarbonyl succinimide in the presence of the alkali and the solvent to obtain a compound (V) that is the N-Fmoc-3-aminopropionic acid derivative. A process route of the N-Fmoc-3-aminopropionic acid derivative is developed in the invention, makes up the blank of the synthesis method of the substance in the prior art, and the synthesis method is simple, few in steps, high in product yield, easily available in adopted raw materials and low in synthesis cost.
Owner:苏州爱玛特生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
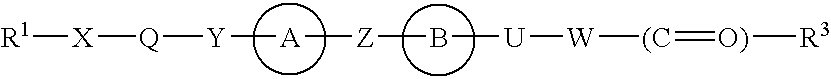
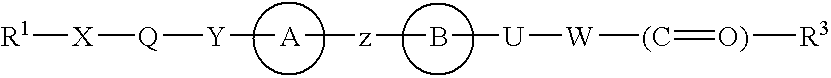
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00001.PNG)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00002.PNG)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/41458c72-594e-4502-b3f4-2bd465aa0ad6/US20160318884A1-20161103-C00003.PNG)

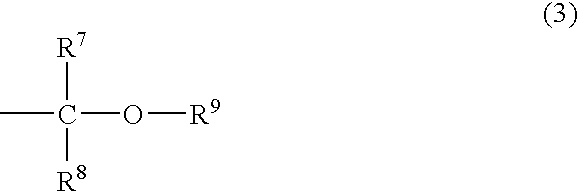
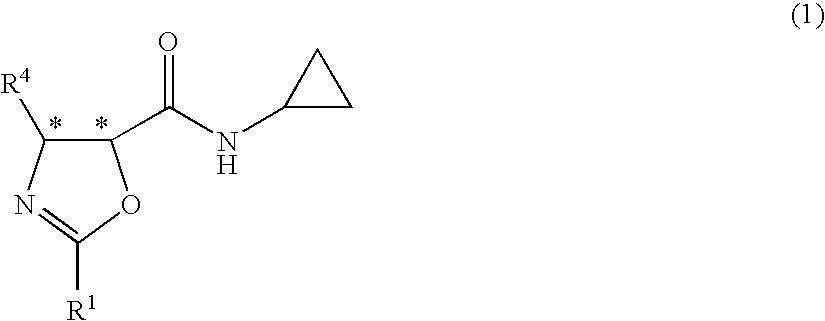
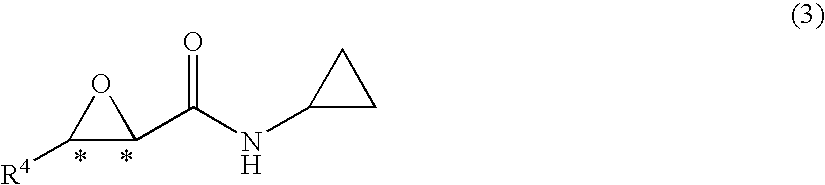
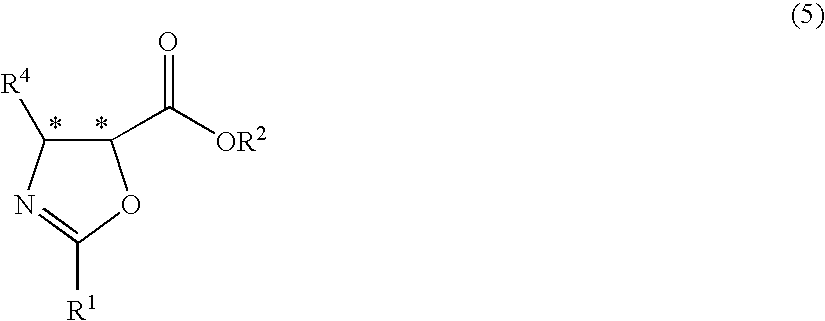
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/24dfd502-e721-44a6-96e8-f358c89223dd/US20190345117A1-C00001.png)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/24dfd502-e721-44a6-96e8-f358c89223dd/US20190345117A1-C00002.png)
![3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors 3-(5-chloro-2-oxobenzo[d]oxazol-3(2H)-yl) propanoic acid derivatives as kmo inhibitors](https://images-eureka.patsnap.com/patent_img/24dfd502-e721-44a6-96e8-f358c89223dd/US20190345117A1-C00003.png)