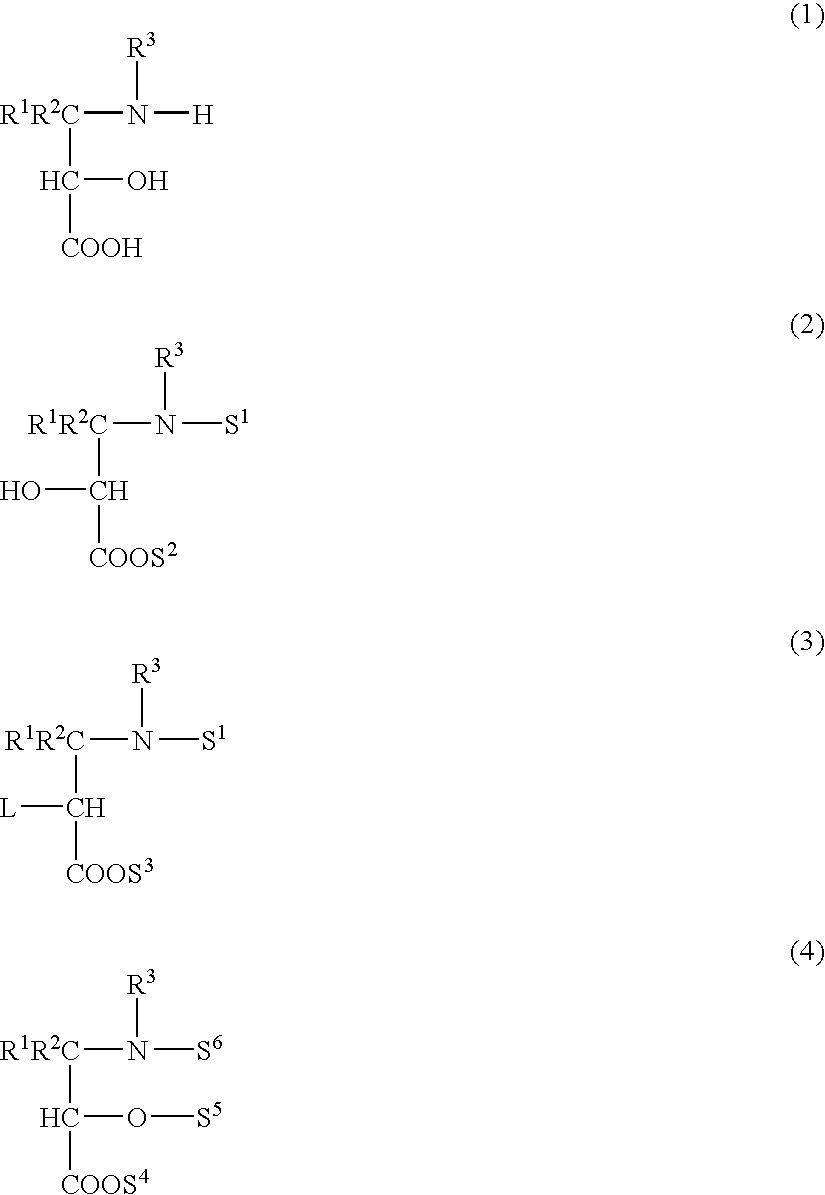Process for producing 3-amino-2-hydroxypropionic acid derivatives
a technology of 3-amino-2-hydroxypropionic acid and derivatives, which is applied in the field of process for preparing 3amino-2-hydroxypropionic acid derivatives, can solve the problems of unsuitable industrial production, process use of phosgene having a very high toxicity as carbonylation agent, and process problems such as unsuitability for industrial production, and achieves stable production, high yield, and reduced yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of (2S,3S)-3-(ethoxycarbonyl)amino-2-hydroxy-4-phenylbutyric acid (erythro-isomer)
[0153] A toluene solution (677.2 g) containing (S)-1,1-dibromo-3-(ethoxycarbonyl)amino-2-oxo-4-phenylbutane (67.65 g) was added dropwise to a 10% aqueous solution of sodium hydroxide (691.3 g) ice-cooled over 10 hours, and stirred at the same temperature for 1 hour. The resultant reaction solution was further reacted at 60° C. for 6 hours. The organic phase was separated from the resultant reaction mixture, ethyl acetate (400 ml) was added to the resultant aqueous phase, and the pH was adjusted to pH 2 with conc. hydrochloric acid (150 g). The organic phase was separated to obtain an aqueous solution (944.7 g) containing erythro-3-amino-2-hydroxy-4-phenylbutyric acid (26.52 g) and threo-3-amino-2-hydroxy-4-phenylbutyric acid (5.44 g). The ratio of erythro-isomer / threo-isomer was 83 / 17, and the diastereomer excess was 66% d.e.
[0154] The aqueous solution (940.4 g) [containing erythro-3-amino-...
example 2
Synthesis of a diastereomer mixture of 3-(ethoxycarbonyl)amino-2-hydroxy-4-phenylbutyric acid
[0156] The ethyl acetate extract (96.5 g) obtained in Example 1 [containing a diastereomer mixture (4.28 g) of 3-(ethoxycarbonyl)amino-2-hydroxy-4-phenylbutyric acid] was concentrated to dry up under reduced pressure. The solid was ground in a porcelain mortar and then further dried in vacuo to obtain a powdery solid (4.43 g). The purity of erythro-3-(ethoxycarbonyl)amino-2-hydroxy-4-phenylbutyric acid was 80% by weight. Also, the HPLC area ratio of erythro-isomer / threo-isomer was 83 / 17, and the diastereomer excess was 66% d.e.
example 3
Synthesis of (2S,3S)-3-(t-butoxycarbonyl)amino-2-hydroxy-4-phenylbutyric acid (erythro-isomer)
[0157] (S)-1,1-Dibromo-3-(t-butoxycarbonyl)amino-2-oxo-4-phenylbutane (42.12 g) was suspended in toluene (420 ml) and water (280 ml) and the suspension was ice-cooled. To this suspension, a 30% aqueous solution of sodium hydroxide (133 g) was added dropwise over 1 hour, and the mixture was stirred at the same temperature for 20 hours. The organic phase was separated from the resultant reaction mixture, ethyl acetate (600 ml) was added to the resultant aqueous phase, the pH was adjusted to pH 2 with conc. hydrochloric acid (115 g), and the aqueous phase was separated. Then, the resultant organic phase was washed with water (70 ml) to obtain an ethyl acetate extract (591.9 g). Determination by HPLC revealed that the amount of the diastereomer mixture of 3-(t-butoxycarbonyl)amino-2-hydroxy-4-phenylbutyric acid in the resultant extract was 27.14 g calculated as an erythro-isomer. Also, the HPL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com