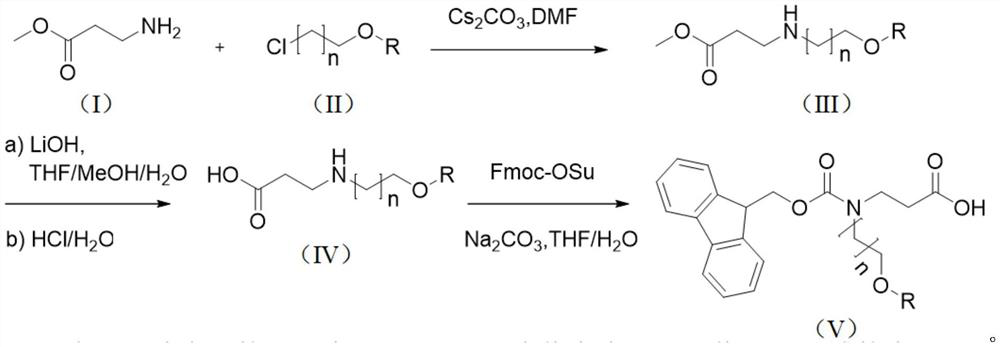
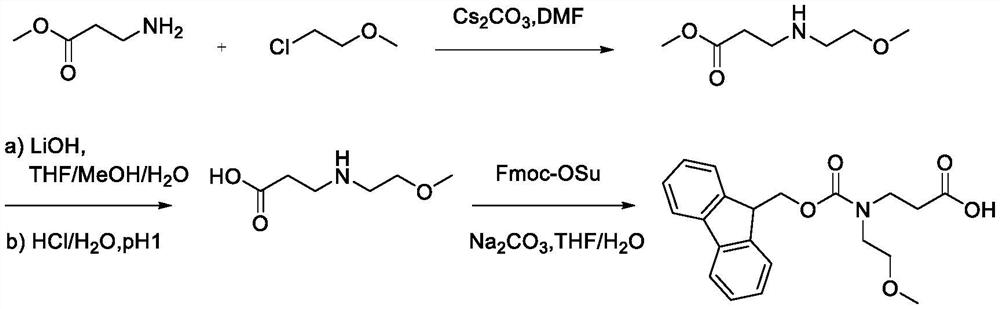
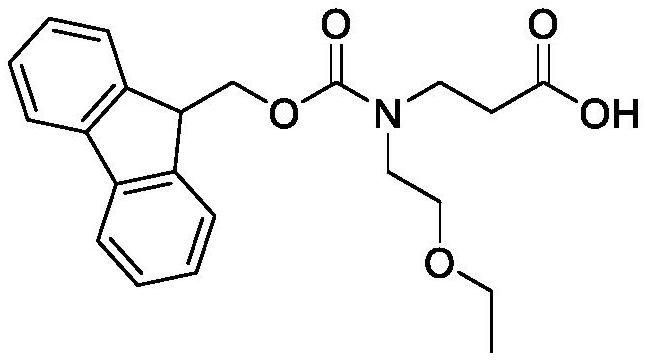
Synthesis method of N-Fmoc-3-aminopropionic acid derivative
A technology for the synthesis of n-fmoc-3-, which is applied in the field of synthesis of N-fmoc-3-aminopropionic acid derivatives, can solve the problems of no reports, etc., and achieve high yield, few steps, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of N-Fmoc-N-2-methoxyethyl-Beta-alanine
[0051]
[0052]The first step: under ice bath conditions, under the protection of argon, (10.3g, 100.00mmol) methyl allanate, (9.27mL, 101.50mmol) chloroethyl methyl ether, (54.2g, 166.40mmol) Cesium carbonate and (200 mL) ultra-dry DMF were added to a 500 mL round bottom flask respectively, and after stirring for 15 minutes, the mixture was heated to room temperature, and stirred for 5 h. After the reaction was completed, extracted with ethyl acetate, dried the organic layer over anhydrous magnesium sulfate, purified by column chromatography, and eluted with ethyl acetate / petroleum ether (V / V=20 / 1) to obtain 3-((2-methoxyethyl (17.33 g, 93%) of methyl)amino)propionate.
[0053] 1 H NMR (400MHz, CDCl 3 )δ3.63(s,3H),3.55(t,2H),3.36(s,3H),3.13(q,2H),2.97(m,2H),2.50(t,2H).
[0054] The second step: Dissolve (10.95g, 67.95mmol) 3-((2-methoxyethyl)amino) propionate methyl ester in (400mL) (V / V / V=4 / 1 / 1) tetrahydrofura...
Embodiment 2
[0058] Synthesis of N-Fmoc-N-2-Ethoxyethyl-Beta-Alanine
[0059]
[0060] The raw material of this embodiment is methyl allanate (100.00mmol) and 2-ethoxyl chloroethane (101.50mmol), other are the same as embodiment 1, the total yield of three-step reaction: 84.9%.
[0061] 1 H NMR(400MHz,DMSO)δ7.92(dd,2H),7.76(m,2H),7.59(td,2H),7.47(td,2H),4.39(d,2H),4.29(td,1H) ,3.69(td,2H),3.56(m,6H),2.70(t,2H),1.15(t,3H).
Embodiment 3
[0063] Synthesis of N-Fmoc-N-3-methoxypropyl-Beta-alanine
[0064]
[0065] The raw material of this embodiment is methyl allanate (100.00mmol) and 3-chloropropyl methyl ether (101.50mmol), and other are the same as embodiment 1, the total yield of three-step reaction: 84.3%.
[0066] 1 H NMR(400MHz,DMSO)δ7.86(dd,2H),7.72(m,2H),7.56(td,2H),7.44(td,2H),4.47(d,2H),4.28(td,1H) ,3.52(t,2H),3.37(dt,4H),3.19(s,3H),2.66(t,2H),1.88(p,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com