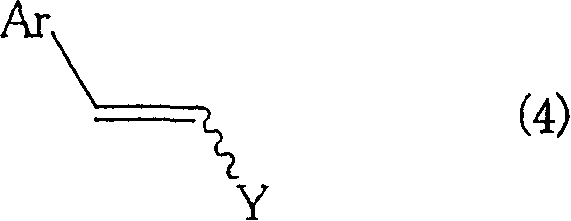
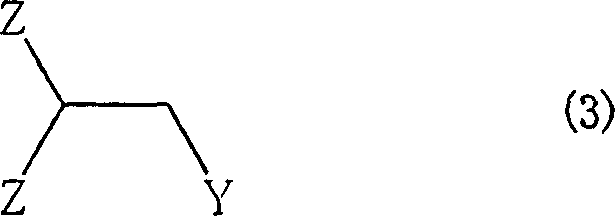Method for producing aromatic unsaturated compound
A compound, unsaturated technology, applied in the field of preparation of aromatic unsaturated compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1.01g 3-(4-fluorophenyl)-1-isopropyl-1H-indole, 0.92g 3,3-dimethoxymethyl propionate, 0.72ml 90% by weight of aqueous acetic acid (containing 4mmol water) and 6 ml of glacial acetic acid were mixed, and then 0.33 g of phosphorus oxychloride was added dropwise to the mixture at an internal temperature of 25° C., and stirred at the same temperature for 9 hours to make it react. After the reaction, 16 ml of water was added dropwise to the reaction liquid, and the precipitated crystals were collected by filtration. The crystals were washed with 20% by volume of aqueous methanol, and then dried to obtain 1.25 g of trans-3-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl ] methyl acrylate (yellow solid). The yield is 93%.
[0078] 1 H-NMR (δ / ppm, CDCl 3 , 400MHz)
[0079] 1.70(6H, d, J=7Hz), 3.76(3H, s), 4.95(1H, m), 5.96(1H, d, J=16Hz), 7.50(1H, d, J=8Hz), 7.57(1H , d, J=8Hz), 7.08-7.40 (6H, m), 7.82 (1H, d, J=16Hz)
Embodiment 2
[0081] Mix 1.04g of 1-methyl-2-phenyl-1H-indole, 0.64g of trans-3-methoxymethyl acrylate, 94.5mg of water and 6ml of glacial acetic acid. To the mixture was added 124 mg of phosphorus oxychloride and stirred at the same temperature for 17 hours to allow a reaction. After the reaction, 30 ml of water was added dropwise to the reaction liquid, and then 50 ml of ethyl acetate was added for extraction, and the obtained organic layer was concentrated. The obtained concentrated residue was purified by flash chromatography to obtain 1.11 g of methyl trans-3-(1-methyl-2-phenyl-1H-indol-3-yl)acrylate (yellow solid). The yield was 76%.
[0082] 1 H-NMR (δ / ppm, CDCl 3 , 400MHz)
[0083] 3.64(3H, s), 3.74(3H, s), 6.46(1H, d, J=16Hz), 7.29-7.55(9H, m), 7.72(1H, d, J=16Hz)
Embodiment 3
[0085] 1.68 g of 1,3,5-trimethoxybenzene, 2.32 g of trans-methyl 3-methoxyacrylate, 0.18 g of water and 6 ml of glacial acetic acid were mixed, and at an internal temperature of 25° C., 164 mg of Phosphorus oxychloride was stirred at the same temperature for 3 hours to make it react. After the reaction was completed, 36 ml of water was added dropwise to the reaction solution, and the precipitated crystals were collected by filtration. The crystals were washed with 20% by volume of aqueous methanol and then dried to obtain 2.28 g of trans-methyl 3-(2,4,6-trimethoxyphenyl)acrylate (white solid). The yield was 91%.
[0086] 1 H-NMR (δ / ppm, CDCl 3 , 400MHz)
[0087] 3.79(3H, s), 3.85(3H, s), 3.87(6H, s), 6.12(2H, s), 6.76(1H, d, J=16Hz), 8.08(1H, d, J=16Hz)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com