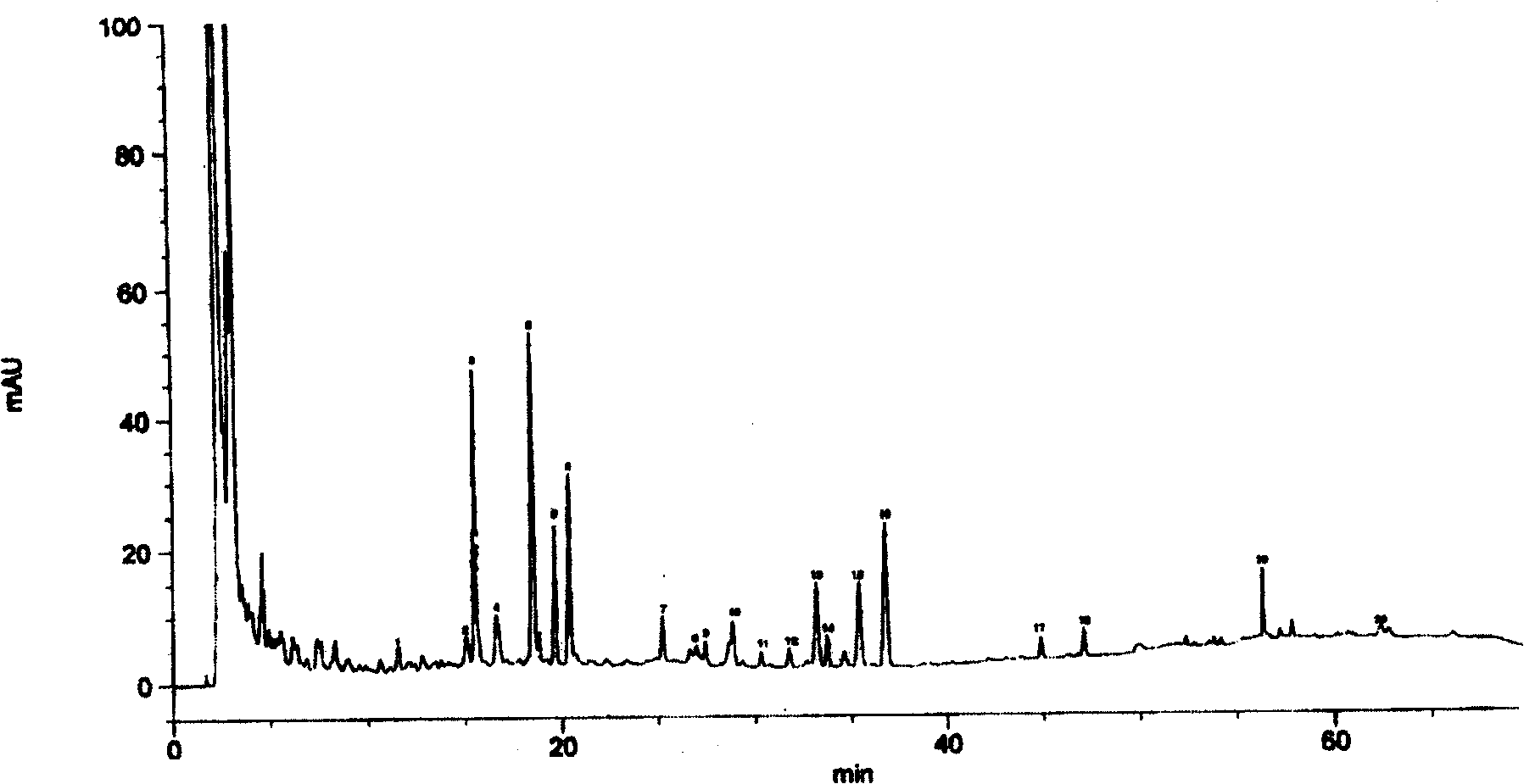
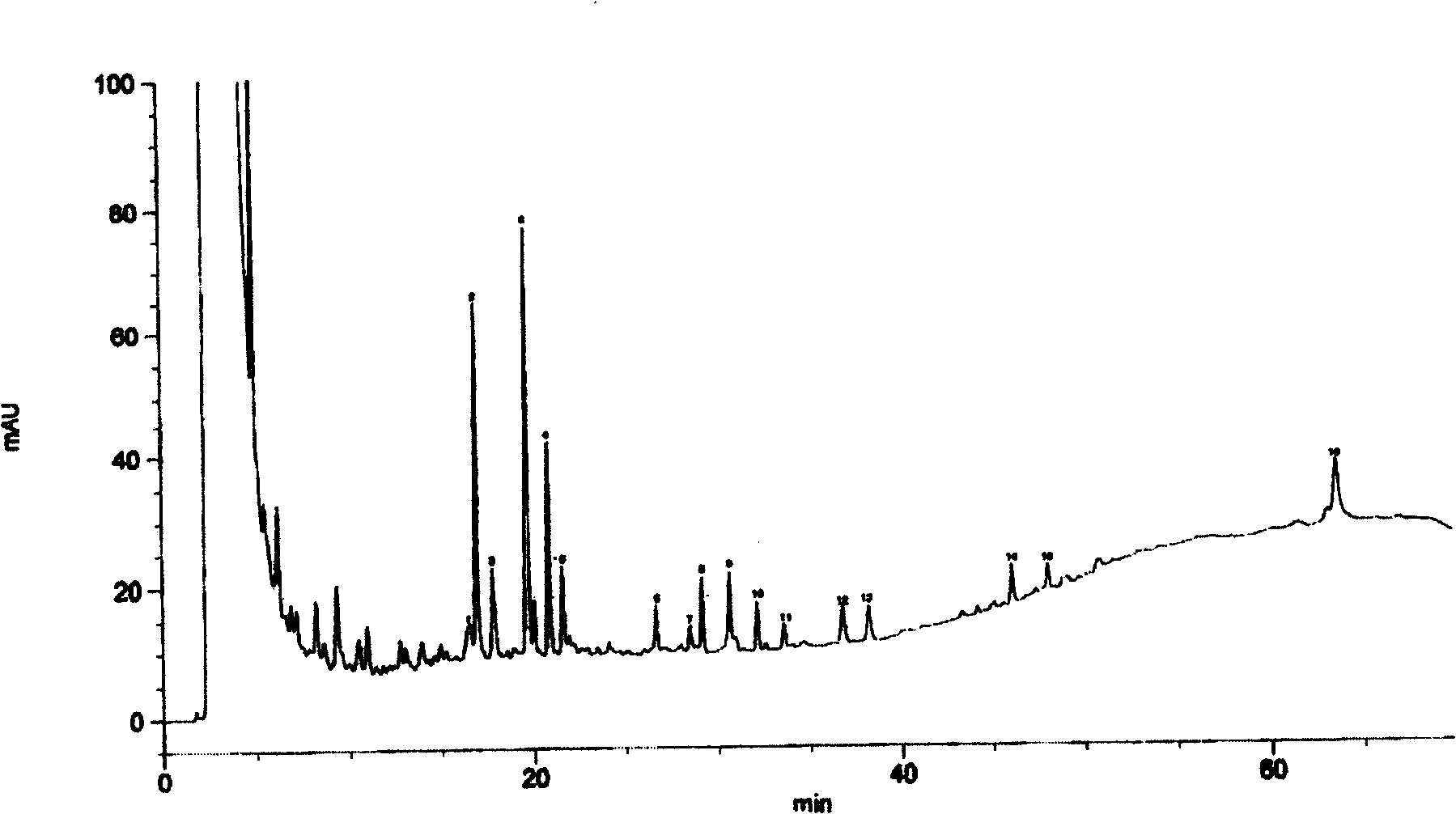
Method for controlling quality of injection for treating tumor
A quality control method and injection technology, which can be applied to antineoplastic drugs, medical preparations containing active ingredients, measuring devices, etc., and can solve the problems of not providing fingerprints, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0066] Experimental Example 1: The Inhibitory Effect of the Injection of the Invention on the Transplanted Tumor Lung Cancer Lewis Sarcoma in Mice
[0067] Take 18~22g healthy pure line C 57 Fifty white mice, half female and half male, were taken from well-grown sarcomas under aseptic conditions, ground with a grinder, diluted with sterile saline 1:2, and subcutaneously inoculated with 0.2ml / mouse under the armpit of the right forelimb. Fasting without water for 17 hours after inoculation, randomly divided into 5 groups according to body weight, 10 in each group, low-dose group, middle-dose group, high-dose group, positive control group, blank control group, the average body weight difference between groups is less than 1g, Immediately after group administration, the tail vein administration was started, once a day, and the blank control group was given the same amount of normal saline, and the administration was continued for 7 days. The animals were killed 24 hours after the...
experiment example 2
[0073] Experimental Example 2: The Inhibitory Effect of the Injection of the Invention on Transplanted Human Small Cell Lung Cancer LTEP-SML in Nude Mice
[0074] Take 50 17-18 BALB / c male nude mice, SPF grade, subcutaneously inoculate the right forelimb axilla with well-grown human lung cancer tumor tissue blocks in nude mice, remove the tumor nodules under aseptic operation in the ultra-clean bench, and take Conventional small block inoculation method, 1 block / mouse (about 1mm 3 ), the tumor inoculation of 50 animals was completed within 40 minutes. When the tumor grows and becomes palpable (1mm 3 ), the animals were randomly divided into 5 groups, 10 in each group, weighed, labeled, low-dose group, middle-dose group, high-dose group, positive control group, blank control group, and the experimental group was administered by tail vein injection every day. Once, the blank control group was given the same amount of normal saline for 15 days, and 24 hours after the last admin...
experiment example 3
[0080] Experimental Example 3: Injection of the present invention to S 180 Synergistic effect of cyclophosphamide chemotherapy on sarcoma (solid type) tumor-bearing mice
[0081] Take 60 healthy Kunming mice of 18-22g, half female and half male, and extract the well-developed S 180 Ascites, diluted with sterile normal saline 1:2, subcutaneously inoculated 0.2ml per mouse under the armpit of the right forelimb. Fasting without water for 17 hours after inoculation, randomly divided into 6 groups according to body weight, 10 rats in each group, low-dose group, middle-dose group, high-dose group, positive control group, model control group, and tumor-bearing blank control group. The average body weight difference between the groups was less than 1 g. After grouping, except the blank control group which was not injected with cyclophosphamide, which was the tumor-bearing control group, all other groups received intraperitoneal injection of cyclophosphamide 30 mg / kg chemotherapy, on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com