Chinese medicine slow-release formulation containing asiatic pennywort herb water-soluble active ingredient, and its preparing method
The technology of a sustained-release preparation and an effective component is applied to the sustained-release preparation of traditional Chinese medicine containing the water-soluble effective component of Centella asiatica and the field of preparation thereof, and can solve the problems of increased toxic and side effects, fluctuation of blood drug concentration, inconvenience for patients to take medicine, and the like, Achieve the effect of delaying release, stabilizing blood concentration and reducing the number of times of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
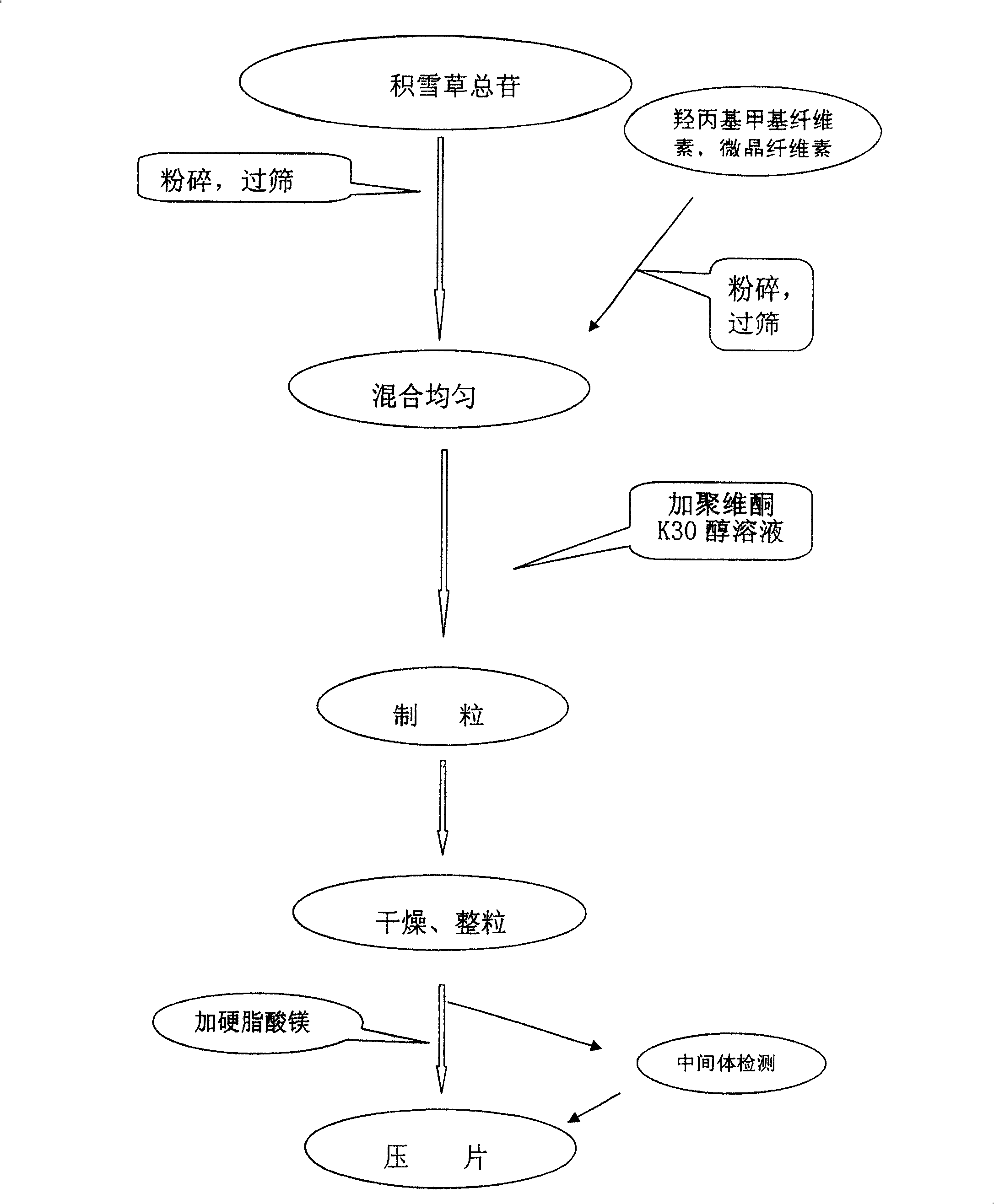
[0030] Weigh the Centella asiatica extract containing 18g of Centella asiaticoside, 30g of hydroxypropyl methylcellulose, and 50g of microcrystalline cellulose, pulverize respectively, mix, add an appropriate amount of povidone K30 alcohol solution, granulate, dry, Whole grains, added with magnesium stearate, compressed into tablets, ready to be obtained.
[0031] In vitro release:
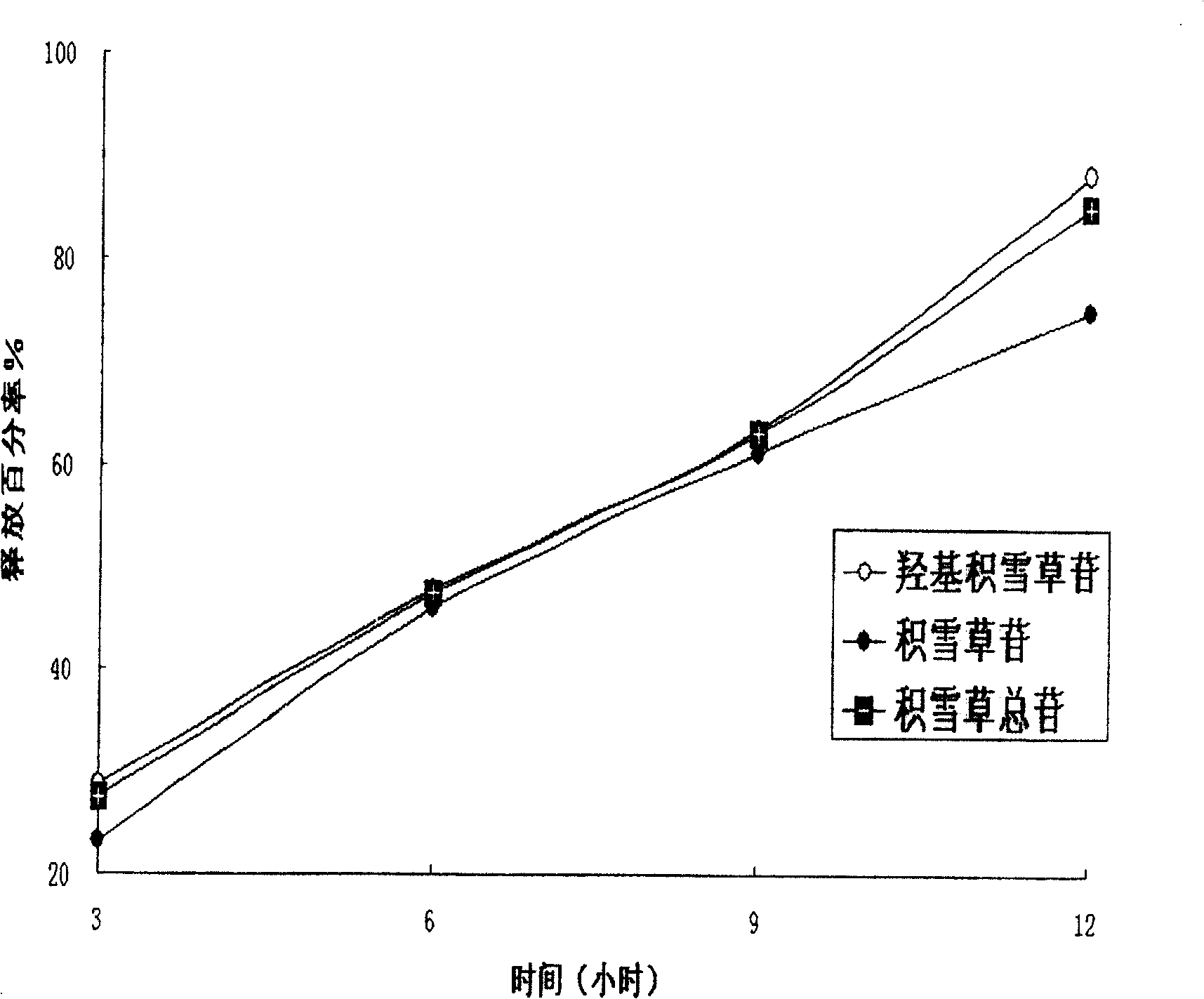
[0032] The results of in vitro drug release studies of sustained-release tablets are shown in figure 2 : It can be seen from this figure that the two main components of asiaticoside and madecassoside, which are the two main components of asiaticoside and madecassoside, can maintain a slow release. In terms of total glycosides, about 28% are released in the 3rd hour, and the drug is released cumulatively in the 6th hour Around 50%, around 65% at the 9th hour, and 85% at the 12th hour. The release conditions are as follows: according to the Chinese Pharmacopoeia 2000 Edition Part II Release Tes...
Embodiment 2
[0034] Weigh the Centella asiatica extract containing 18g of Centella asiaticoside, 225g of hydroxypropyl methylcellulose, and 50g of microcrystalline cellulose, pulverize respectively, mix, add an appropriate amount of povidone K30 alcohol solution, granulate, dry, Whole grains, add 1 g of magnesium stearate, and compress into tablets.
[0035] In vitro release:
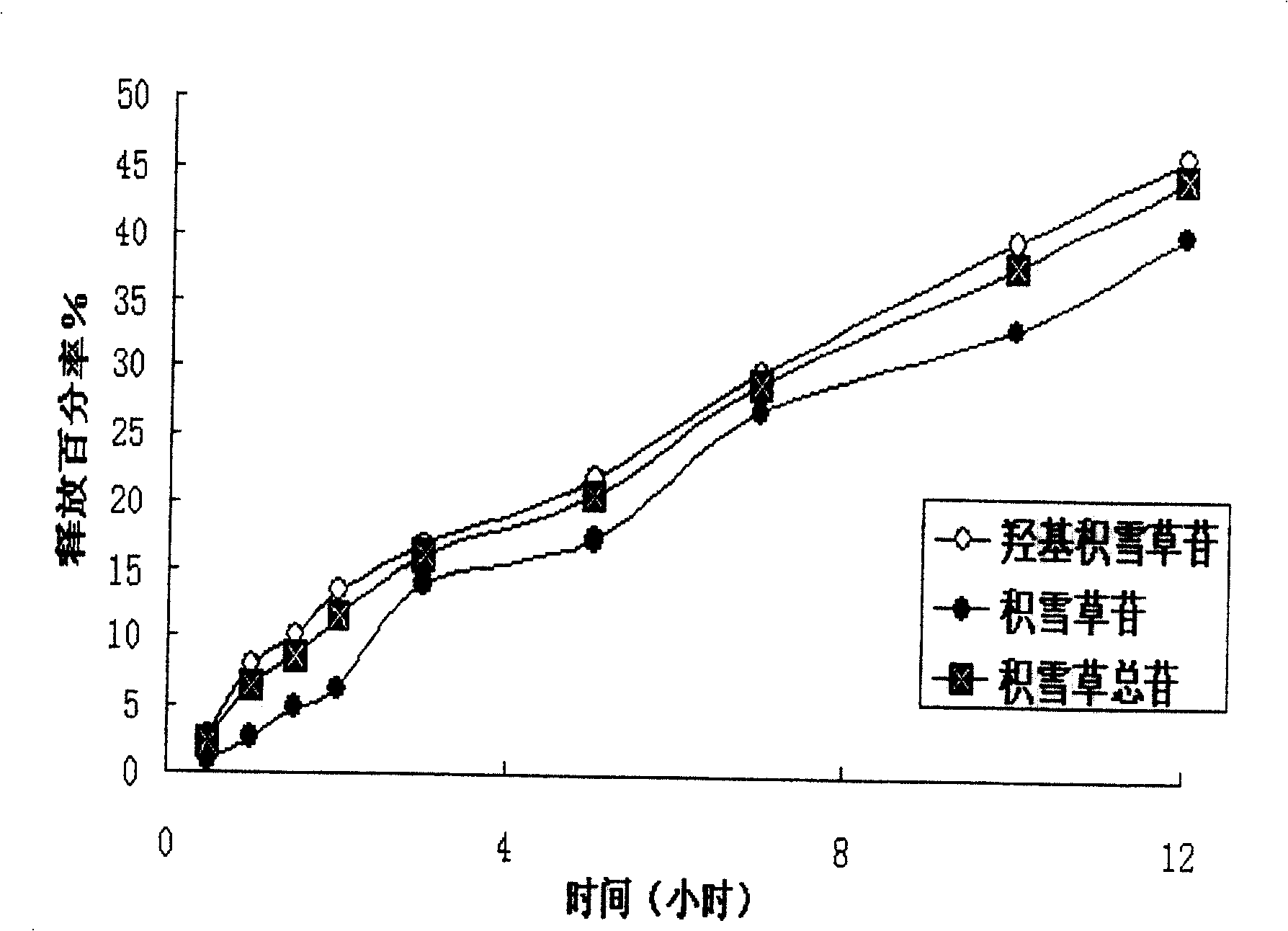
[0036] The results of in vitro drug release studies of sustained-release tablets are shown in image 3 : It can be seen from this figure that the two main components of asiaticoside, madecassoside and madecassoside, can maintain a slow release. In terms of total glycosides, about 15% is released in the 3rd hour, and the drug is released cumulatively in the 5th hour Around 20%, around 38% at the 10th hour, and 45% at the 12th hour. The release conditions are as follows: according to the Chinese Pharmacopoeia 2000 Edition Part II Release Test Method (Blue Method), using water as the dissolution medium, temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com