Determination of inorganic phosphorus and its diagnostic kit
A diagnostic kit and inorganic phosphorus technology are applied in the field of medical testing and determination, which can solve the problems affecting the use and promotion, the difference in the accuracy of the sensitivity test results, etc., and achieve the effects of low test cost, easy popularization and application, and accurate test results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment one (single agent)
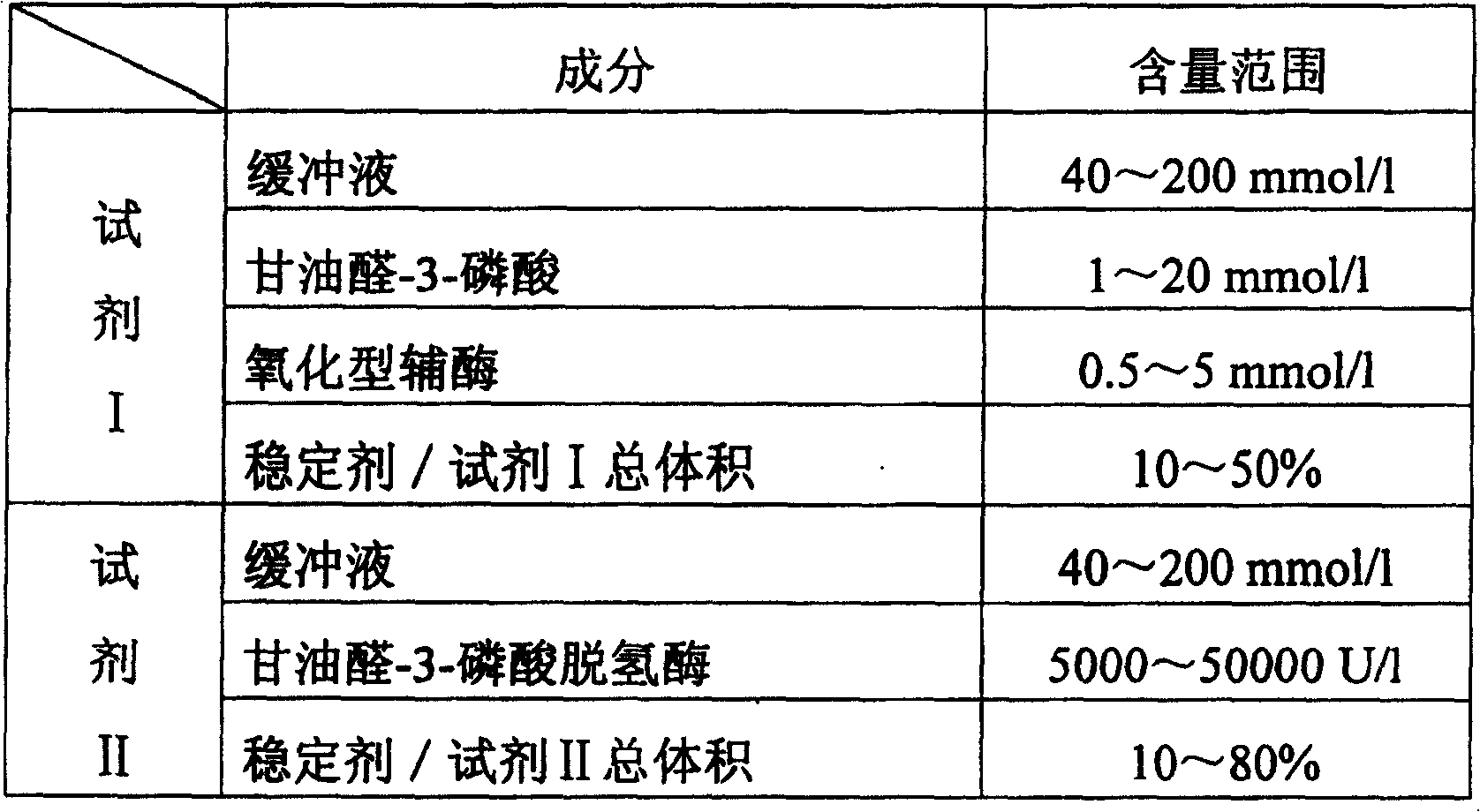
[0046] Prepare the diagnostic kit for inorganic phosphorus in serum according to the following components and dosage:
[0047] Glycine buffer 80mmol / l,
[0048] Glyceraldehyde-3-phosphate 2mmol / l,
[0049] thio-NAD + 1mmol / l,
[0050] Glyceraldehyde-3-phosphate dehydrogenase 10000U / l,
[0051] Ethylene glycol 50% (accounting for the total volume of the reagent).
[0052] Set on the automatic biochemical analyzer (Hitachi-7080): temperature 37°C, reaction time 10 minutes, test main wavelength 340nm, test secondary wavelength 405nm or more, the volume ratio of the tested sample to the reagent is 1:25, and the reaction direction It is a positive reaction (absorbance rises, the same below).
[0053] Add the serum sample and the prepared single agent, and the two are automatically mixed in the analyzer to detect and record the increase of the absorbance at 340nm. A total of 31 reading points were detected, and the data was r...
Embodiment 2
[0054] Embodiment two (two doses)
[0055] Prepare the diagnostic kit for inorganic phosphorus in serum according to the following components and dosage:
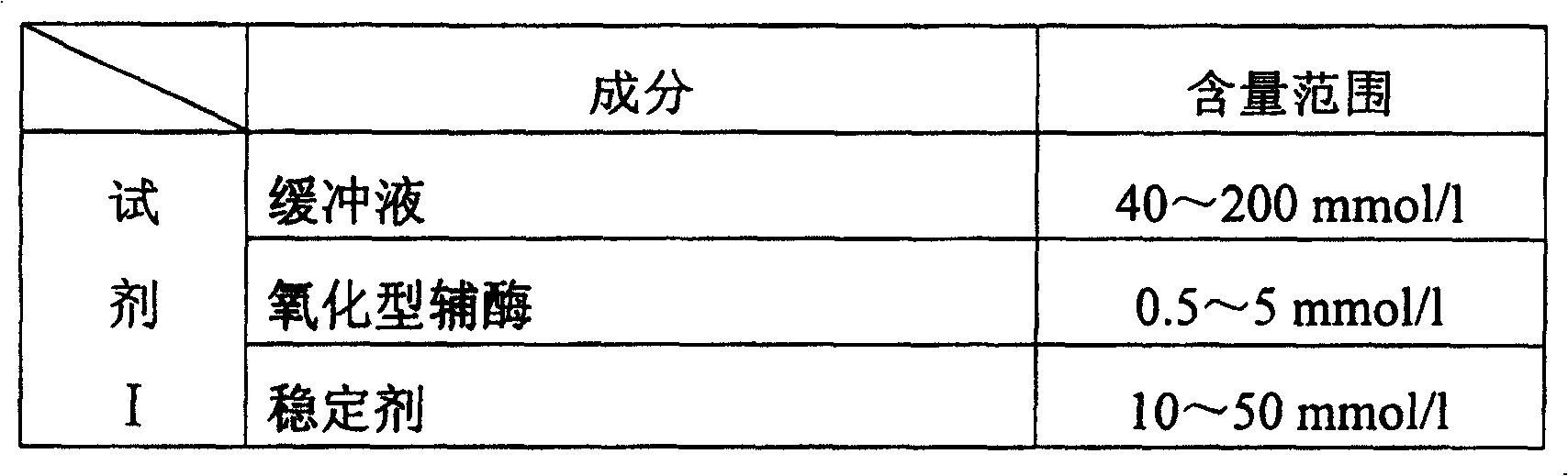
[0056] Reagent I——
[0057] Imidazole ~ hydrochloric acid buffer 100mmol / l,
[0058] Glyceraldehyde-3-phosphate 6mmol / l,
[0059] NADP + 2mmol / l,
[0060] Glycerin 20mmol / l
[0061] Reagent II——
[0062] Imidazole ~ hydrochloric acid buffer 100mmol / l,
[0063] Glyceraldehyde-3-phosphate dehydrogenase 20000U / l,
[0064] Ethylene glycol 50% (accounting for the total volume of reagent II).
[0065] Set on the automatic biochemical analyzer (Hitachi-7080): temperature 30°C, reaction time 15 minutes, test main wavelength 340nm, test secondary wavelength 405nm or more, the ratio of the total volume of the tested sample to reagent I and reagent II is 1:25, the ratio of the amount of reagent I to reagent II is 4:1, and the reaction direction is positive reaction.
[0066] Serum sample and reagent I were add...
Embodiment 3
[0067] Embodiment three (three doses)
[0068] Prepare the diagnostic kit for inorganic phosphorus in serum according to the following components and dosage:
[0069] Reagent I——
[0070] Triethanolamine buffer 120mmol / l,
[0071] thio-NADP + 4mmol / l,
[0072] Propylene glycol 50% (accounting for reagent I total volume);
[0073] Reagent II——
[0074] Triethanolamine buffer 120mmol / l,
[0075] Glyceraldehyde-3-phosphate 10mmol / l,
[0076] Propylene glycol 50% (accounting for the total volume of reagent II);
[0077] Reagent III——
[0078] Triethanolamine buffer 120mmol / l,
[0079] Glyceraldehyde-3-phosphate dehydrogenase 30000U / l,
[0080] Ethylene glycol 50% (accounting for the total volume of reagent III).
[0081] Set on the automatic biochemical analyzer (Hitachi-7080): temperature 25°C, reaction time 20 minutes, test main wavelength 340nm, test secondary wavelength 405nm or more, the total volume of the tested sample and reagent I, reagent II and r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com