Freeze dried ginseng polysaccharide powder for injection and its preparing process
A technology of ginseng polysaccharide and freeze-dried powder injection, which is applied in the direction of freeze-drying transportation, medical preparations containing active ingredients, powder transportation, etc., can solve the problems of poor preparation effect of ginseng polysaccharide raw materials, and achieve reliable and large-scale drug quality. Market space, the effect of high content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
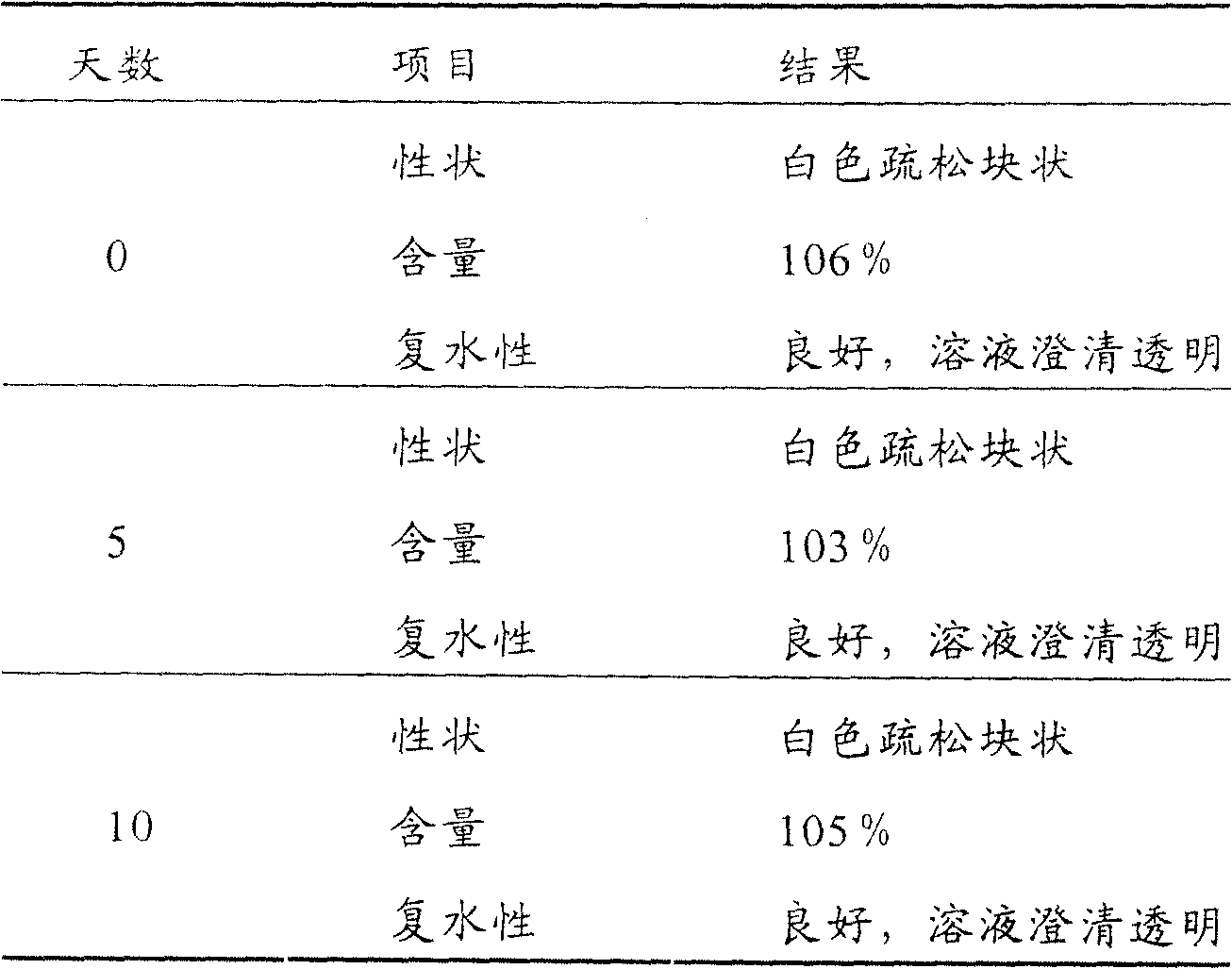
Examples
Embodiment 1
[0036] Embodiment one: the extraction of ginseng polysaccharide
[0037]The extraction method of ginseng polysaccharide of the present invention comprises the following steps:
[0038] A) Collect the root of fresh ginseng, wash it, crush it, pass it through a 80-100 mesh sieve, soak it in water overnight, dry it, and soak it with 75-80% ethanol for extraction or reflux extraction at least 5 times;
[0039] B) After volatilizing the dregs to remove all ethanol, add water to soak, heat to boiling, keep slightly boiling for extraction, and extract at least 5 times in total;
[0040] C) Combine the extracts, concentrate to 1 / 10 volume, add 95% ethanol or absolute ethanol until the alcohol concentration is 40%, centrifuge at 3000rpm for 15 minutes, discard the precipitate, and continue to add 95% ethanol or absolute ethanol to the supernatant , until the alcohol concentration is 60%, centrifuge at 3000rpm for 15 minutes, collect the precipitate, dissolve it in water, refrigerate o...
Embodiment 2
[0044] Example 2: Preparation of ginseng polysaccharide freeze-dried powder for injection
[0045] The freeze-dried ginseng polysaccharide powder for injection of the present invention includes ginseng polysaccharide and water-soluble auxiliary materials for injection, and its raw material components are: 3% ginseng polysaccharide, 5% dextran, 92% injection use water. The preparation steps are as follows: (1) weigh 30 g of ginseng polysaccharide and 50 g of dextran under aseptic conditions, place them in a sterile container, add 100 g of water for injection to dissolve, and make a material solution; % Add active carbon to the material solution, stir, and filter; (3) heat the filtrate in a water bath to 50°C, place it at 30-50°C for 60 minutes, then add 820g of water for injection; (4) filter with a 0.22μm microporous membrane, check pH and ginseng polysaccharide content, after passing the inspection, they were aseptically divided into vials and half-added with butyl rubber st...
Embodiment 3
[0046] Example 3: Preparation of ginseng polysaccharide freeze-dried powder for injection
[0047] The freeze-dried ginseng polysaccharide powder for injection of the present invention includes ginseng polysaccharide and water-soluble auxiliary materials for injection, and its raw material components are: 0.3% ginseng polysaccharide, 5% mannitol, and 94.7% injection Water, its preparation steps are: (1) take by weighing ginseng polysaccharide 3g, mannitol 50g under aseptic condition, place in aseptic container, add 100g water for injection to dissolve, make material solution; (2) by weight volume percentage: 0.3% activated carbon was added to the material solution, stirred, and filtered; (3) the filtrate was heated to 50°C in a water bath, placed at 35-45°C for 50 minutes, and then 845g of water for injection was added; (4) filtered with a 0.22 μm microporous membrane, Check the pH and ginseng polysaccharide content, after passing the inspection, aseptically dispense them into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com