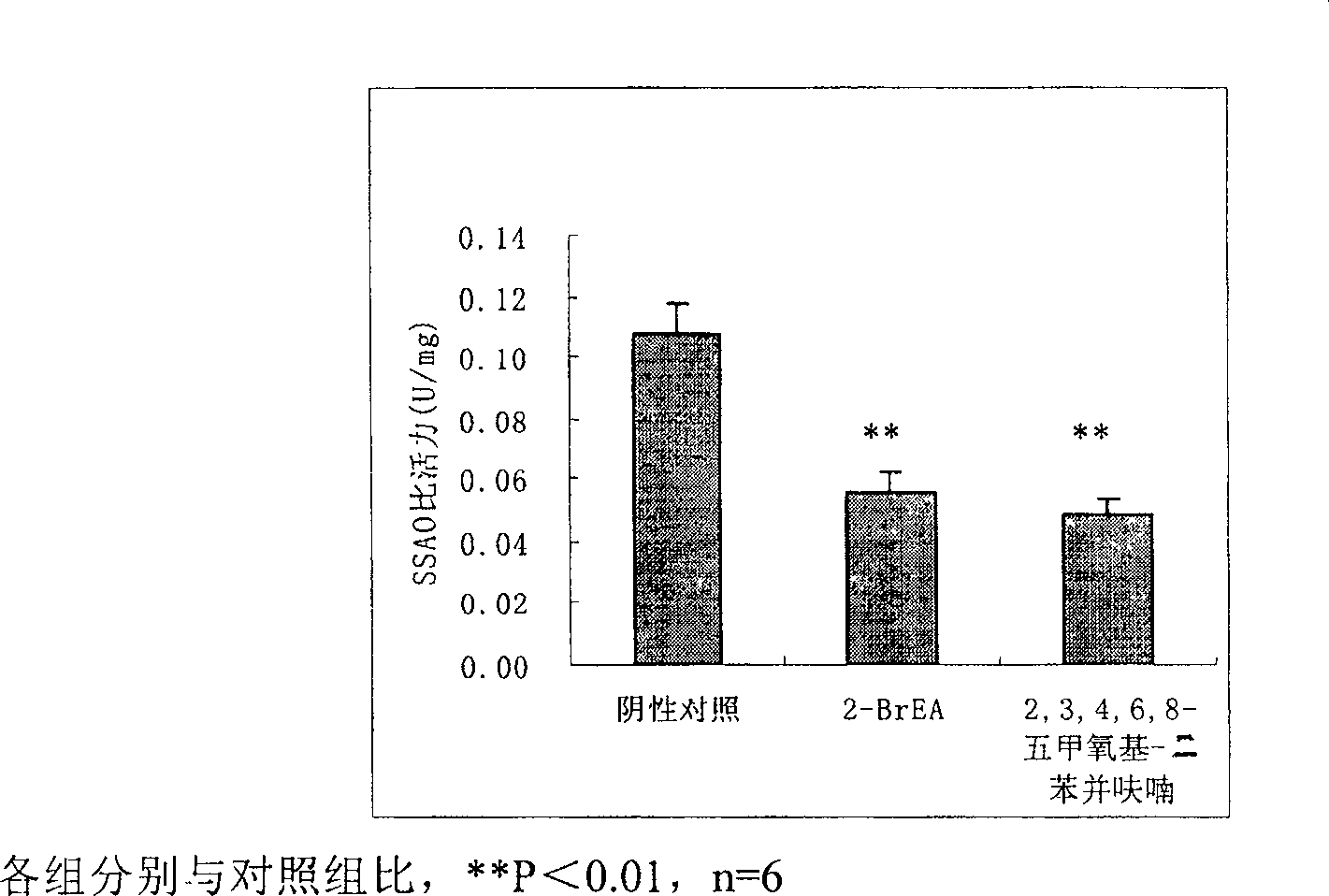
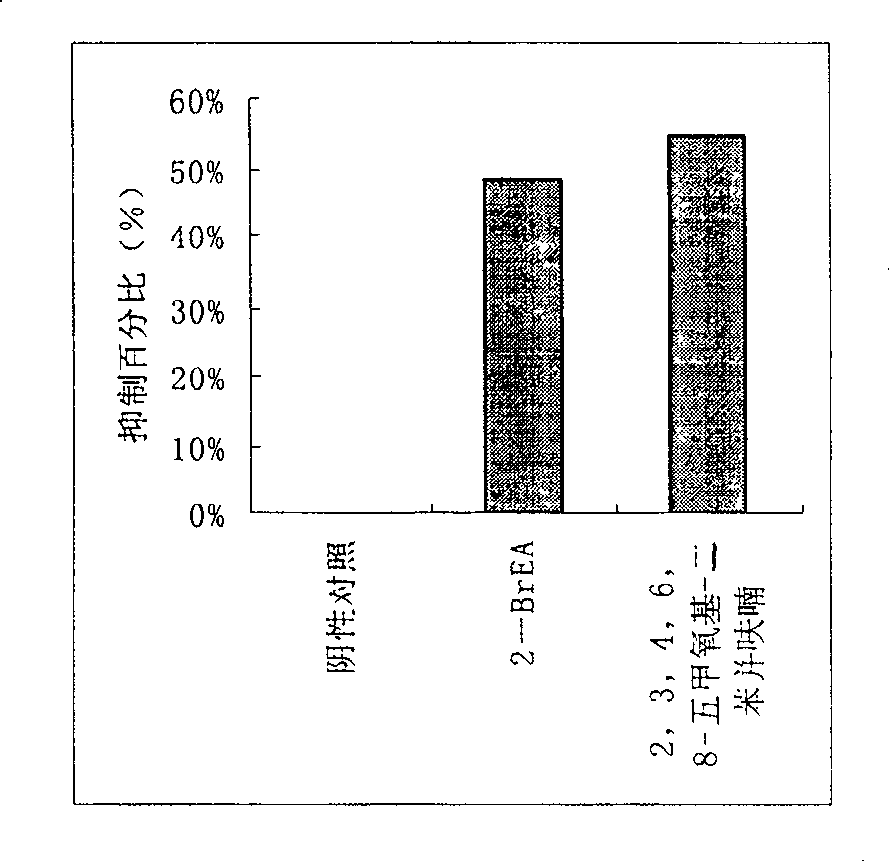
Compound 2,3,4,6,8-pentamethoxyl-dibenzofuran and its use
A pentamethoxy, diphenyl technology, applied in the field of natural medicinal chemistry, can solve problems such as toxicity, and achieve significant social and economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of 2,3,4,6,8-pentamethoxy-dibenzofuran
[0040] (1) 5Kg of the root of the natural plant Rosaceae Hawthorn plant Hawthorn is weighed and pulverized, and the pulverization refers to pulverization by a common pulverizer;
[0041] (2) adding 50L of 80% ethanol to reflux extraction of crushed plant fragments for 3 hours to obtain ethanol extract;
[0042] (3) The extract is filtered with a clarification plate, the filtrate is recovered under vacuum and reduced pressure to remove the solvent, the weight of the obtained extract is weighed to be 0.5Kg, and the extract is pulverized into fine powder by an ordinary pulverizer;
[0043] (4) add petroleum ether, chloroform, ethyl acetate successively to extract by the weight percentage of extract and petroleum ether, chloroform, ethyl acetate is 1:20, the petroleum ether of gained, chloroform extraction solution adopts vacuum decompression recovery to remove solvent respectively , to obtain the crude product;
[0...
Embodiment 2
[0089] 1. Preparation of 2,3,4,6,8-pentamethoxy-dibenzofuran
[0090] (1) 5Kg of the root of the natural plant Rosaceae Hawthorn plant Hawthorn is weighed and pulverized, and the pulverization refers to pulverization by a common pulverizer;
[0091] (2) adding 50L of 95% ethanol to reflux extraction of crushed plant fragments for 3 hours to obtain ethanol extract;
[0092] (3) The extract is filtered with a clarification plate, the filtrate is recovered under vacuum and decompression to remove the solvent, and the weight of the obtained extract is weighed to be 0.45Kg, and the extract is pulverized into fine powder by an ordinary pulverizer;
[0093] (4) Add petroleum ether, chloroform, and ethyl acetate to extract in sequence according to the weight percentage of the extract and petroleum ether, chloroform, and ethyl acetate at 1:20, and wherein the petroleum ether and chloroform extracts obtained are respectively removed by vacuum decompression recovery Solvent, obtain crud...
Embodiment 3
[0097] 1. Preparation of 2,3,4,6,8-pentamethoxy-dibenzofuran
[0098] (1) 5Kg of the root of the natural plant Rosaceae Hawthorn plant Hawthorn is weighed and pulverized, and the pulverization refers to pulverization by a common pulverizer;
[0099] (2) adding the crushed plant fragments to 60L water for reflux extraction for 3 hours to obtain a water extract;
[0100] (3) The extract is filtered with a clarification plate, the filtrate is recovered under vacuum and decompression to remove the solvent, and the weight of the obtained extract is weighed to be 0.38Kg, and the extract is pulverized into fine powder by an ordinary pulverizer;
[0101] (4) Add petroleum ether, chloroform, and ethyl acetate to extract in sequence according to the weight percentage of the extract and petroleum ether, chloroform, and ethyl acetate at 1:20, and wherein the petroleum ether and chloroform extracts obtained are respectively removed by vacuum decompression recovery Solvent, obtain crude pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com