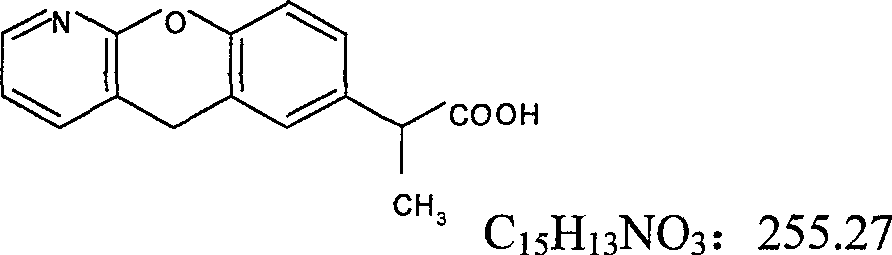
New pranoprofen eye drops and its preparation method
A technology of eye drops and aqueous solutions, applied in the field of medicine, can solve the problems of unseen pranoprofen eye drops, etc., and achieve stable and reliable storage effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] prescription:
[0019] Pranoprofen 1g
[0020] Sodium Hyaluronate 1g
[0021] Appropriate amount of related accessories
[0022] Add water for injection to 1000ml
[0023] Preparation method:
[0024] Get pranoprofen and related auxiliary materials and dissolve in appropriate amount of water for injection respectively.
[0025] Take the prescribed sodium hyaluronate in water for injection, stir continuously at 60-70°C to make it fully swell, boil, and filter while hot with a microporous membrane.
[0026] Slowly add the filtered sodium hyaluronate solution into the pranoprofen solution, stir evenly while adding, add water for injection to a sufficient amount, filter to obtain a clear solution, subpackage, and inspect the quality to complete.
Embodiment 2
[0028] prescription:
[0029] Pranoprofen 1g
[0030] Sodium Hyaluronate 1g
[0031] Boric acid 15g
[0032] Appropriate amount of related accessories
[0033] Add water for injection to 1000ml
[0034] Preparation method:
[0035] Get pranoprofen, boric acid and related auxiliary materials and dissolve in appropriate amount of water for injection respectively.
[0036] Take the prescribed sodium hyaluronate in water for injection, stir continuously at 60-70°C to make it fully swell, boil, and filter while hot with a microporous membrane.
[0037] Slowly add the filtered sodium hyaluronate solution into the pranoprofen solution, stir evenly while adding, add water for injection to a sufficient amount, filter to obtain a clear solution, subpackage, and inspect the quality to complete.
Embodiment 3
[0039] prescription:
[0040] Pranoprofen 1g
[0041] Sodium Hyaluronate 2g
[0042] Boric acid 15g
[0043] Appropriate amount of related accessories
[0044] Add water for injection to 1000ml
[0045] Preparation method:
[0046] Get pranoprofen, boric acid and related auxiliary materials and dissolve in appropriate amount of water for injection respectively.
[0047] Take the prescribed sodium hyaluronate in water for injection, stir continuously at 60-70°C to make it fully swell, boil, and filter while hot with a microporous membrane.
[0048] Slowly add the filtered sodium hyaluronate solution into the pranoprofen solution, stir evenly while adding, add water for injection to a sufficient amount, filter to obtain a clear solution, subpackage, and inspect the quality to complete.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com