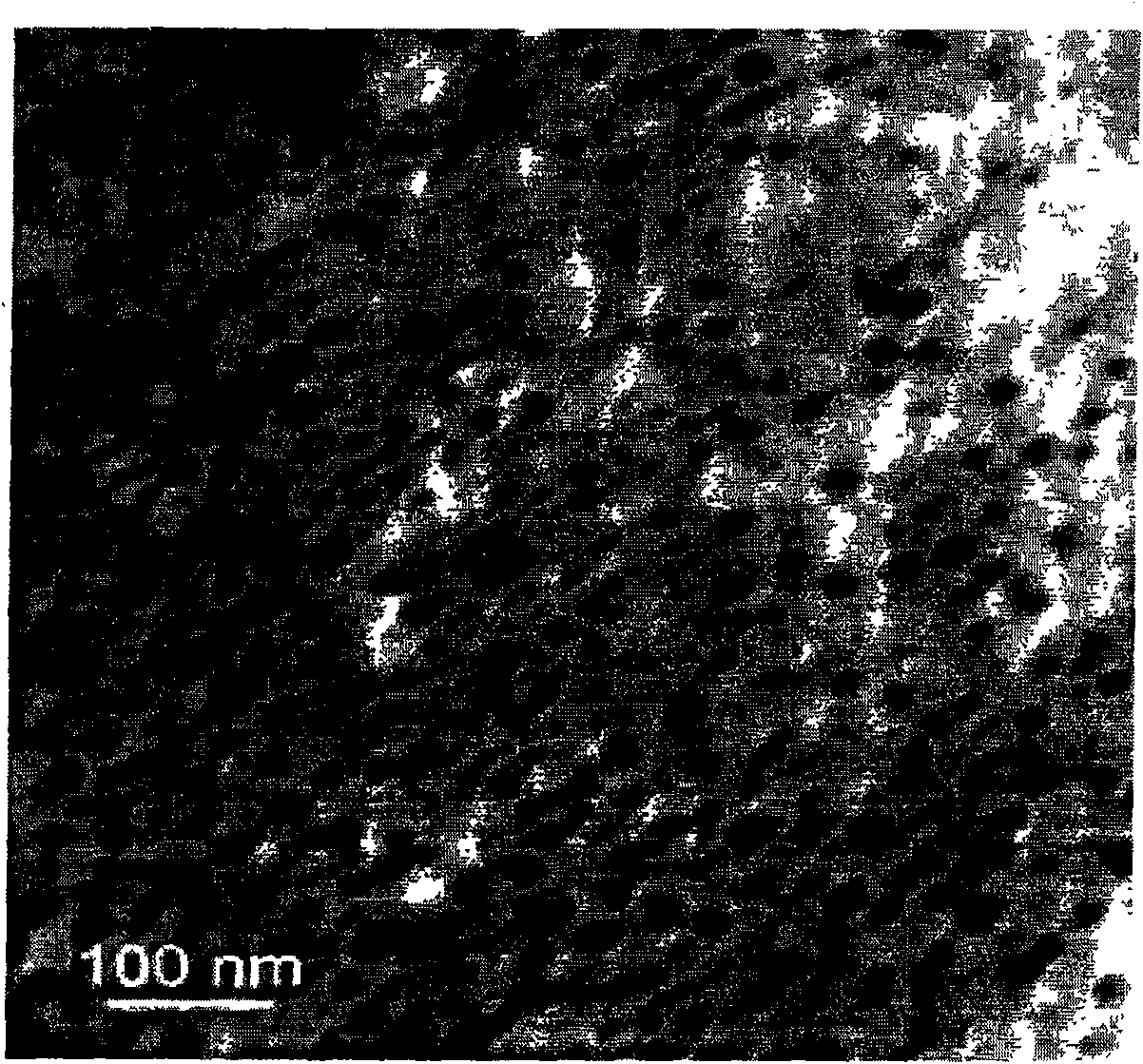
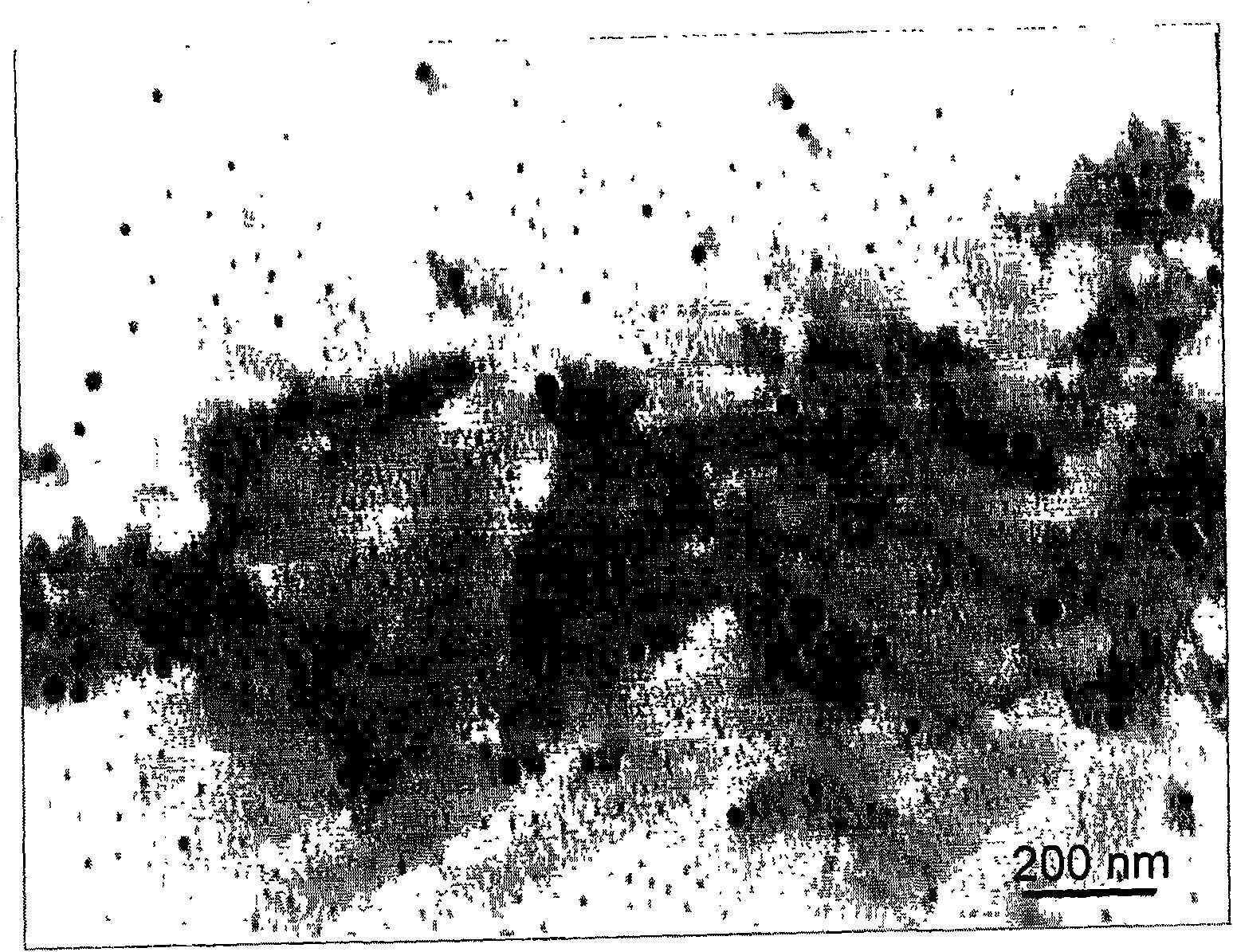
Method of synthesizing poly-pyrrole nano partical by diphenylamine sulfonic acid copolymerization method
A technology of polypyrrole nanometer and diphenylamine sulfonic acid, which is applied in the field of in-situ preparation of polypyrrole nanoparticle synthesis, can solve the problems of cumbersome preparation and removal process, damage to physical and mechanical properties, and influence on product purity, so as to overcome molding processing problems, The effect of simplifying the synthesis process and the simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 2.1702g of sodium diphenylamine sulfonate and add it to a 250mL glass bottle, then add 75mL of hydrochloric acid solution with a concentration of 1.0mol / L, stir to form a diphenylamine sulfonic acid solution after the reaction is complete, then measure 0.145mL of pyrrole monomer and add Add it to the diphenylamine sulfonic acid solution, stir evenly, seal the glass bottle and place it in a 10°C reaction water bath, and keep the temperature constant for more than half an hour under vigorous stirring. 0.8111g ferric chloride (FeCl 3 ) into an 80mL glass bottle, then add 25mL of hydrochloric acid solution with a concentration of 1.0mol / L, and stir to fully dissolve it; ferric chloride (FeCl 3 ) solution into a separatory funnel, and drop into the monomer solution at a rate of 1 drop / 3 seconds to initiate polymerization. After the oxidant was added dropwise, the reaction was continued for 24 hours to complete the reaction. After the reaction was completed, a dark gr...
Embodiment 2
[0025] Weigh 1.3563g of sodium diphenylamine sulfonate into a 250mL glass bottle, then add 75mL of hydrochloric acid solution with a concentration of 0.1mol / L, stir and react to form a diphenylamine sulfonic acid solution, then measure 0.3617mL of pyrrole monomer and add Add it to the diphenylamine sulfonic acid solution, stir evenly, seal the glass bottle and place it in a 10°C reaction water bath, and keep the temperature constant for more than half an hour under vigorous stirring. 0.8111g ferric chloride (FeCl 3 ) into an 80mL glass bottle, then add 25mL of hydrochloric acid solution with a concentration of 0.1mol / L, stir to fully dissolve it, transfer the ferric chloride solution into a separatory funnel, and drop into the monomer at a rate of 1 drop / 3 seconds In solution, polymerization is initiated. After the ferric chloride solution was added dropwise, the reaction was continued for 24 hours to make it react completely. After the reaction is completed, a dark green poly...
Embodiment 3
[0027] Weigh 2.1702g of sodium diphenylamine sulfonate and add it to a 250mL glass bottle, then add 75mL of hydrochloric acid solution with a concentration of 0.1mol / L, stir to form a diphenylamine sulfonic acid solution after the reaction is complete, then measure 0.145mL of pyrrole monomer and add Add it to the diphenylamine sulfonic acid solution, stir evenly, seal the glass bottle and place it in a 10°C reaction water bath, and keep the temperature constant for more than half an hour under vigorous stirring. Add 1.1410g of ammonium persulfate into an 80mL glass bottle, then add 25mL of hydrochloric acid solution with a concentration of 0.1mol / L, stir to dissolve it fully, transfer the ammonium persulfate solution into a separatory funnel, and pour the ammonium persulfate solution at a speed of 1 drop / 3 seconds Drop into the monomer solution to initiate polymerization. After the ammonium persulfate solution was added dropwise, the reaction was continued for 24 hours to comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com