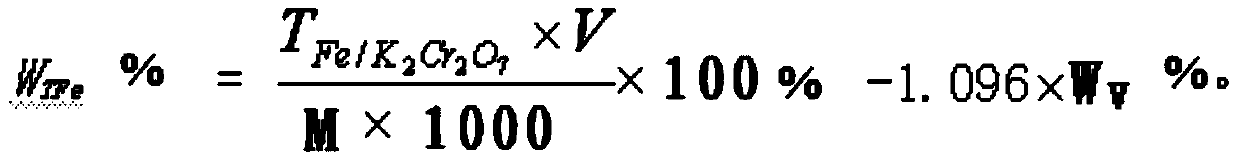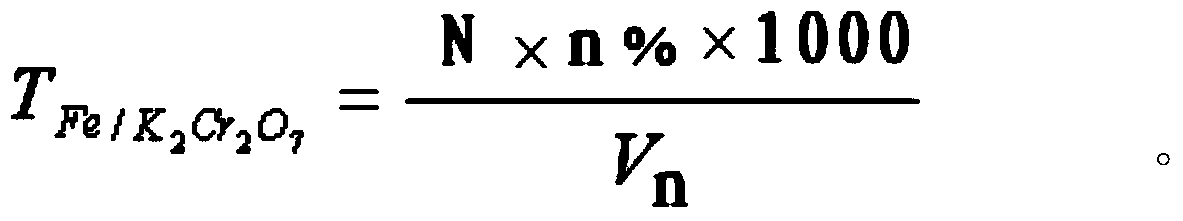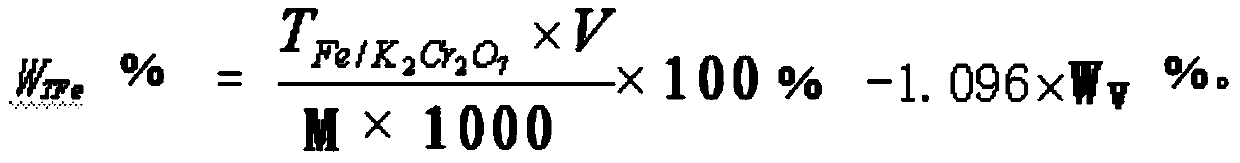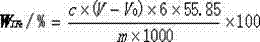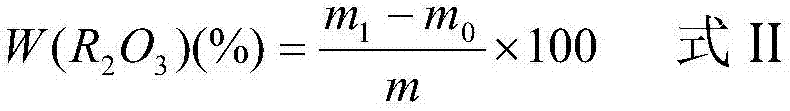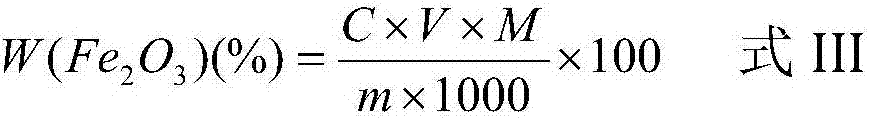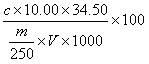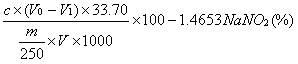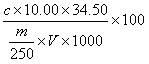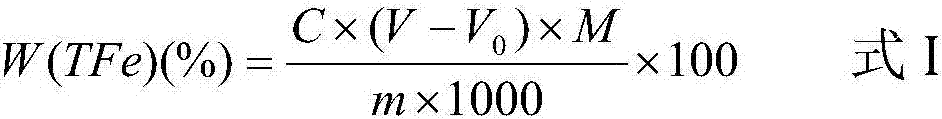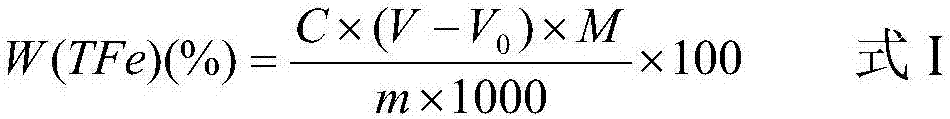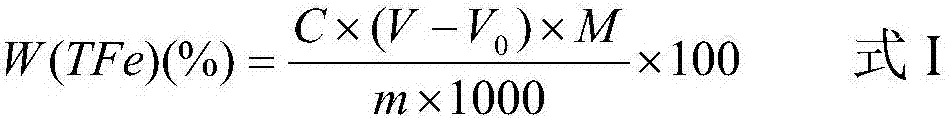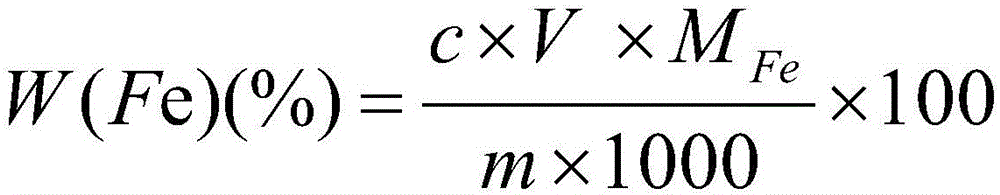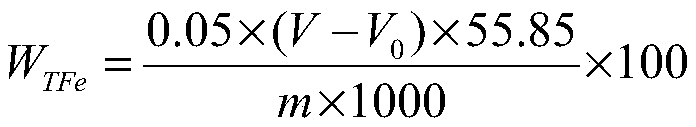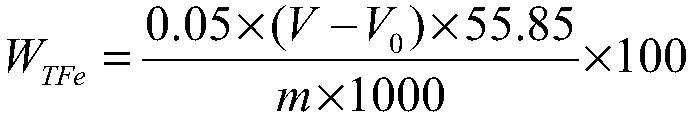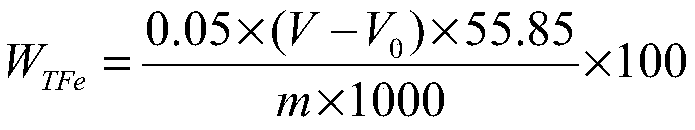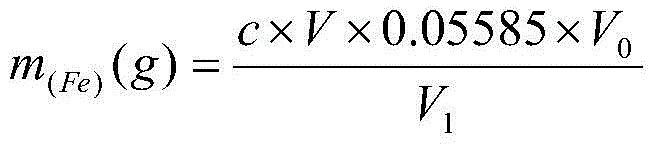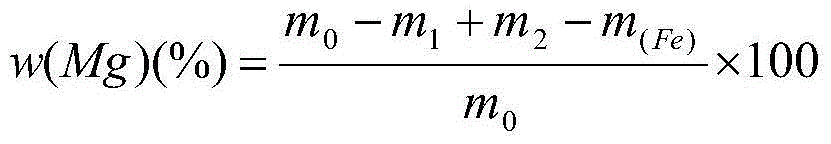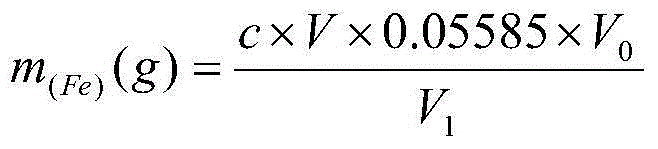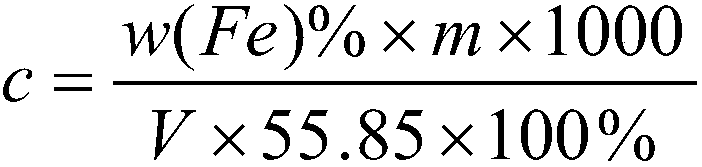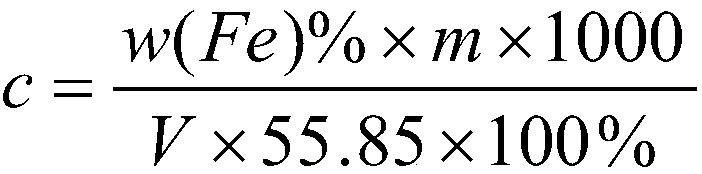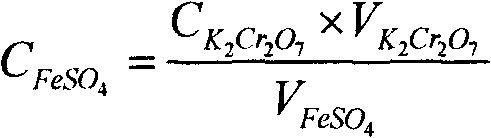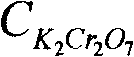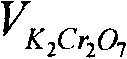Patents
Literature
62 results about "Sodium diphenylamine sulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
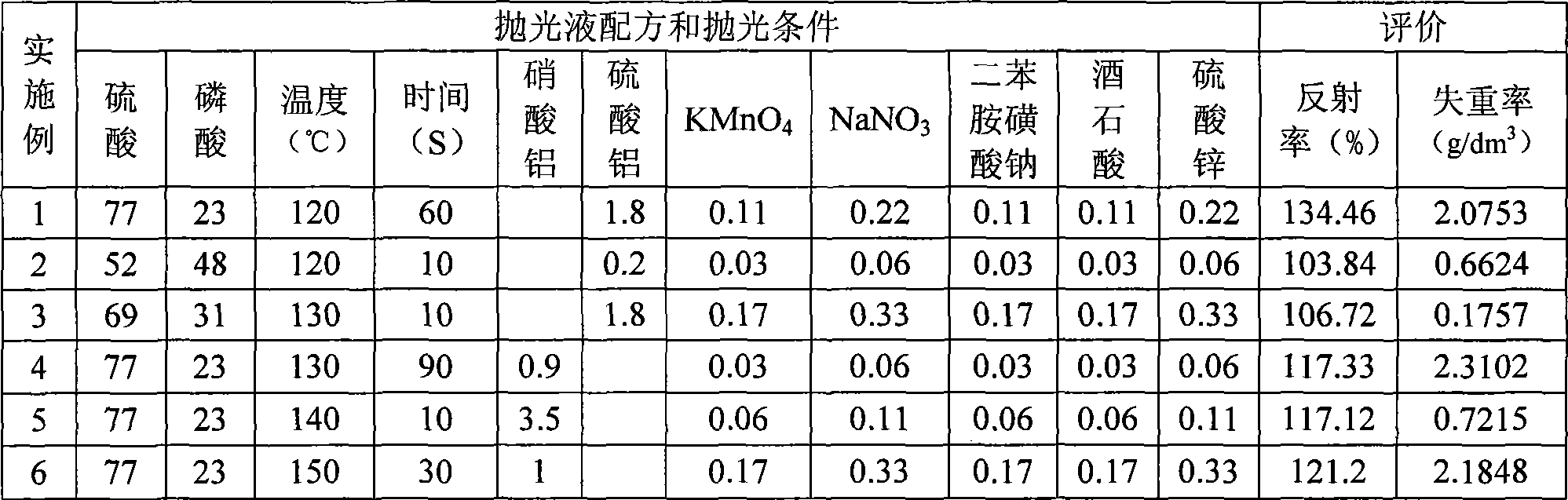
Aluminum product chemical polishing solution and polishing method thereof
InactiveCN101476126AEasy to cleanReduce corrosion pointsPhosphoric acidSodium diphenylamine sulfonate
The invention discloses an aluminium product chemical polishing solution composition with nitric acid free. The composition uses sulfuric acid and phosphoric acid as a basic solution, an additive is composed of aluminium sulphate (or aluminium nitrate), potassium permanganate, sodium nitrate, diphenylamine sulfonic acid sodium salt, tartaric acid and zinc sulfate. Polishing temperature is 110-150 DEG C, polishing time is 10s-120s when the polishing solution is used for polishing. The composition can ensure no nitrogen oxide yellow smoke harm, at the same time ensure the aluminium product having better polishing blare effect for reaching mirror surface brightness, and having little rate of weight loss.
Owner:SHANXI UNIV
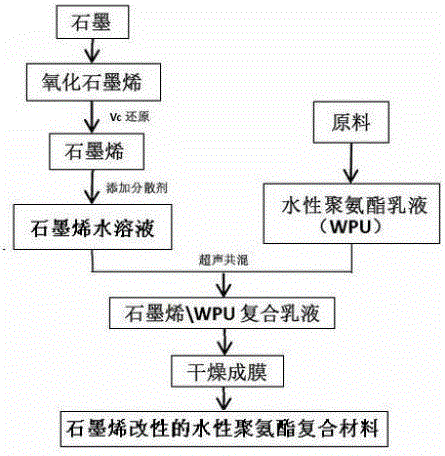
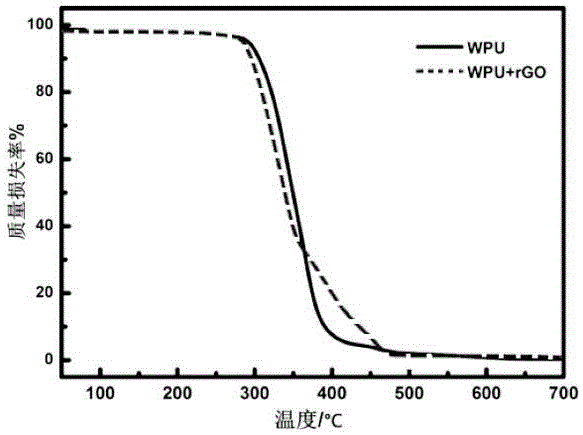
Preparing method of graphene modified waterborne polyurethane composite material
The invention relates to a preparing method of a graphene modified waterborne polyurethane composite material. The preparing method includes the following steps that (1) a prepolymer is prepared; (2) waterborne polyurethane emulsion is prepared; (3) a graphene aqueous solution is prepared, wherein firstly, graphene oxide is prepared, secondly, graphene reduced by vitamin C is prepared, and thirdly, the graphene aqueous solution is prepared; and (4) the graphene modified waterborne polyurethane composite material is prepared. The prepolymer of polyether glycol and toluene diisocynate obtained after water removal is adopted for preparing the waterborne polyurethane; sodium diphenylaminesulfonate is added into the graphene reduced by the vitamin C to prepare the graphene aqueous solution; and then the waterborne polyurethane and the graphene aqueous solution are subject to ultrasonic dispersion and are treated to obtain the graphene modified waterborne polyurethane composite material. The preparing method is simple and convenient, the cost is low, and high efficiency and environment protection are achieved, the graphene modified waterborne polyurethane composite material is suitable for large-scale production, and very good application prospects on the fields of antistatic protection, building coatings and the like are achieved.
Owner:EAST CHINA UNIV OF SCI & TECH
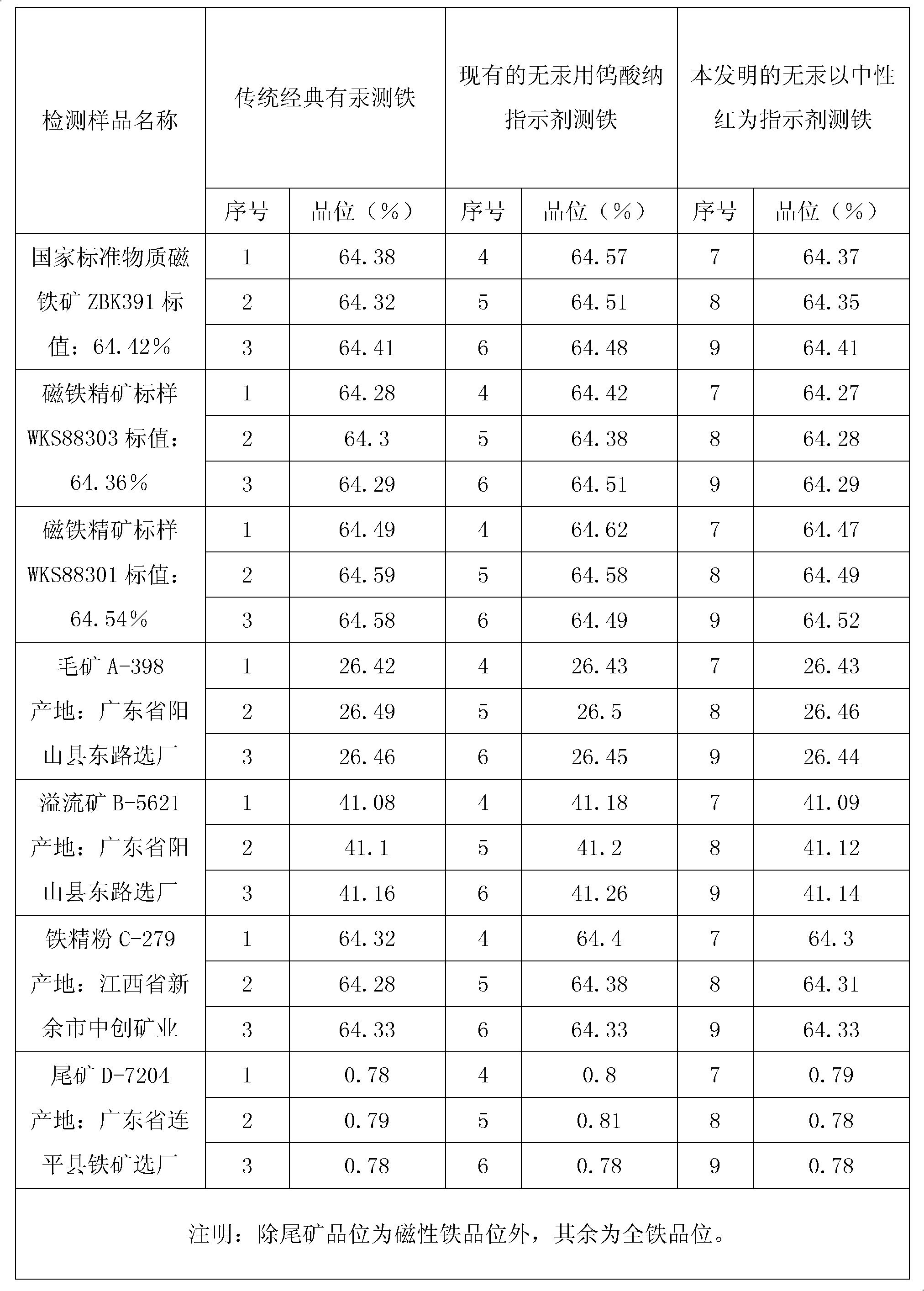
Novel method for measuring iron without mercury
InactiveCN102608112AEliminate pollutionGood reproducibilityMaterial analysis by observing effect on chemical indicatorPhosphoric acidColor changes
The invention relates to a novel method for measuring iron without mercury, which comprises the following steps of: decomposing a test sample through mixed acid of hydrochloric acid, sulfuric acid and phosphoric acid; taking neutral red and sodium diphenylaminesulfonate as indicators; measuring the iron through a SnCl2-TiCl3-K2Cr2O7 volumetric method; reducing Fe<3+> of phi Fe<3+> / Fe<2+>=+0.76V through tin bichloride with phi Sn<4+> / Sn<2+>=+0.16V; adding neutral red reagent when the solution is flavescent, i.e. the solution still contains a small amount of Fe<3+>; taking the color change of the neutral red as an indicator indicating that Fe<3+> is completely reduced to be Fe<2+> according to the principle that the neutral red is reduced to be colorless after the Fe<3+> is reduced completely, and the neutral red reagent is blue in the Fe<3+> solution; dropping titanium trichloride with phi Ti<4+> / Ti <3+>=+0.1V until blue is disappeared; removing excess titanium trichloride through oxidation by a 5.000g. L<-1> sodium diphenylaminesulfonate-potassium dichromate standard solution method; and finally, calculating ion grade of the test sample.
Owner:JIANGXI UNIV OF SCI & TECH
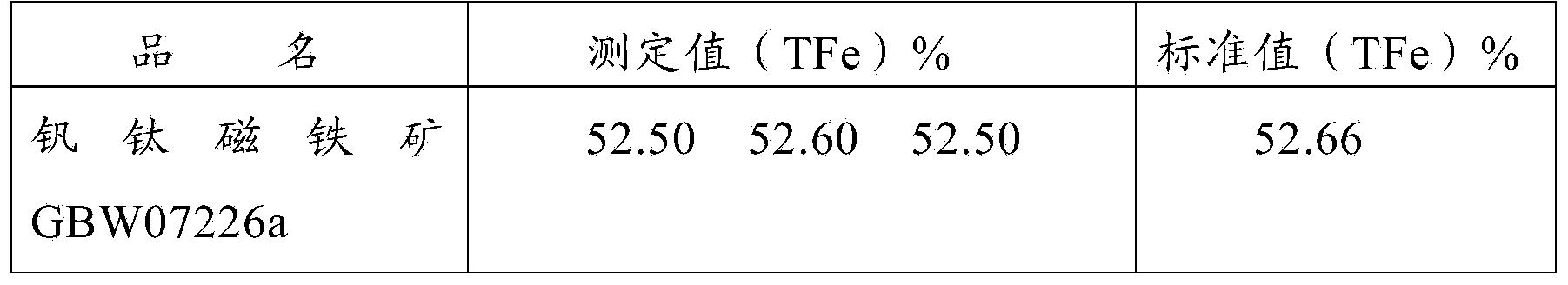
Method for rapidly determining content of total iron in vanadium titano-magnetite
InactiveCN104181272AEasy to operateQuick breakdownMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationMagnetiteRoom temperature
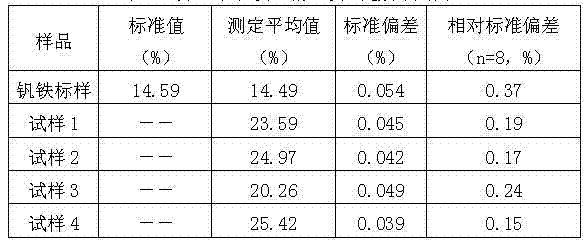
The invention provides a method for rapidly determining the content of total iron in vanadium titano-magnetite. The method comprises the following steps: (1) weighing a to-be-determined vanadium titano-magnetite sample with the weight of M; (2) adding sufficient mineral dissolving solution to dissolve the to-be-determined vanadium titano-magnetite sample in a heating manner; (3) adding dilute hydrochloride acid into a reaction vessel; (4) dropwise adding stannous chloride solution to the reaction vessel until the mixed solution turns pale yellow, reheating the mixed solution to be boiled slightly, and cooling the mixed solution to the room temperature; (5) adding sodium tungstate solution into the reaction vessel, dropwise adding titanium trichloride solution until the mixed solution turns blue, and dropwise adding titanium trichloride solution to be slightly excessive in volume; (6) dropwise adding potassium dichromate solution into the reaction vessel until the mixed solution is colorless, and dropwise adding sodium diphenylaminesulfonate indicator; (7) titrating the mixed solution in the reaction vessel to be purple by adopting potassium dichromate standard titration solution; (8) sampling the solution after being titrated to be purple, and determining the weight percentage of vanadium in the sample; and (9) calculating. The method is easy to operate, high in determination speed, capable of enabling the detection result to be equivalent to that of a national standard method and capable of better meeting the in-situ rapid analysis requirement.
Owner:武汉钢铁有限公司
Method of synthesizing poly-pyrrole nano partical by diphenylamine sulfonic acid copolymerization method
InactiveCN101033294AOvercoming Forming Processing ProblemsEasy to preparePolypyrroleSodium diphenylamine sulfonate
The invention discloses a method for synthesizing polypyrrole nano-particles with diphenylamine sulfoacid, its characters are as follow: it prepares the monomer solution with the pyrrole and diphenylamine sodium in the acidic solution in accordance with a molar ratio of 10:90 ~ 90:10, and then it adds the oxidant solution to the monomer solution for reaction. In which, the molar ratio of the pyrrole and diphenylamine sodium with the oxidizer are 1: 0.1 ~ 1.
Owner:TONGJI UNIV
Method for determining total iron by titanium trichloride
InactiveCN102519958AEasy to operateAccurate measurementMaterial analysis by observing effect on chemical indicatorToxic materialSodium tungstate
The invention belongs to the technical field of methods for determining total iron and particularly relates to a method for determining total iron by titanium trichloride. The invention aims to solve the technical problems and provide the method for determining the total iron by the titanium trichloride, which has simple and convenient process without discharging toxic substances. The method for determining the total iron by the titanium trichloride comprises the following steps: (1) melting a sample and then leaching the smelt sample by acid; (2) by adopting sodium tungstate as an indicator, adding the titanium trichloride into the leached solution until tungsten blue is generated, and dropping potassium dichromate solution until the blue is disappearer; and (3) by adopting sodium diphenylamine sulfonate as an indicator, performing titration with potassium dichromate standard solution, and calculating the content of the total iron according to consumption volume of the potassium dichromate standard solution. The method for titration of the total iron by the titanium trichloride has the advantages of more accurate measuring result, higher qualified rate and less analytical error.
Owner:攀枝花钢企米易白马球团有限公司
Method for determining total iron in vanadium titano-magnetite by using acid dissolution method
InactiveCN104391077AReduce operating proceduresReduce manpower expenditureChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorPhosphateAcid dissolution
The invention relates to a method for determining total iron in vanadium titano-magnetite by using an acid dissolution method. The method comprises the following steps: dissolving a sample with sulfuric acid-phosphate mixed acid; in a hydrochloric acid medium, using stannous chloride to reduce a great amount of ferric iron, using potassium permanganate to oxidize vanadium, using sodium tungstate as an indicator, using titanium trichloride to reduce ferric iron to diatomic iron, oxidizing excessive titanium trichloride with potassium dichromate until tungsten blue fades, using sodium diphenylaminesulfonate as an indicator, and determining the content of iron with potassium dichromate titration. Compared with determination with an alkali fusion method, the method greatly shortens the operating process, saves drugs, reduces human resource cost, and improves the production efficiency; as the potassium permanganate oxidation process is adopted, the interference of vanadium on iron is eliminated, the determination results are stable, and data is accurate.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Method for rapidly determining content of total iron in nitrided ferrovanadium
InactiveCN103091450AGuaranteed accuracyDetection speedChemical analysis using titrationPreparing sample for investigationFerric hydroxideBoiling point
The invention discloses a method for rapidly determining the content of total iron in nitrided ferrovanadium. The method comprises the steps of: 1, decomposing a nitrided ferrovanadium sample by an alkali fusion method to obtain an alkali fusion sample; 2, leaching the alkali fusion sample by hydrochloric acid, adding sodium hydroxide to a leaching solution for precipitation, and filtering and separating the solution to obtain a ferric hydroxide precipitate; 3, acidifying the ferric hydroxide participate by hydrochloric acid, heating the acidified precipitate to a boiling point, dropwise adding stannous chloride to the heated precipitate to reduce ferric iron into ferrous until the stannous chloride is excessive; and 4, oxidizing the excessive stannous chloride by mercuric chloride, titrating the oxidized stannous chloride by a potassium dichromate standard solution with sodium diphenylaminesulfonate being an indicator, thus calculating the content of the total iron of the nitrided ferrovanadium sample. The method is capable of separating vanadium and ferrum and eliminating interference of high vanadium to determination of the total iron, thereby ensuring the accuracy of a determination result. The method is used for directly determining the content of the total iron in the nitrided ferrovanadium, the detection time is shortened from original 10h to 1h, and the detection speed is greatly enhanced. The method has the characteristics of being direct, simple, convenient and feasible.
Owner:HEBEI IRON AND STEEL
Preparation method of graphene
ActiveCN102862978AContains less impurity elementsEasy to controlGrapheneSodium diphenylamine sulfonateReducing agent
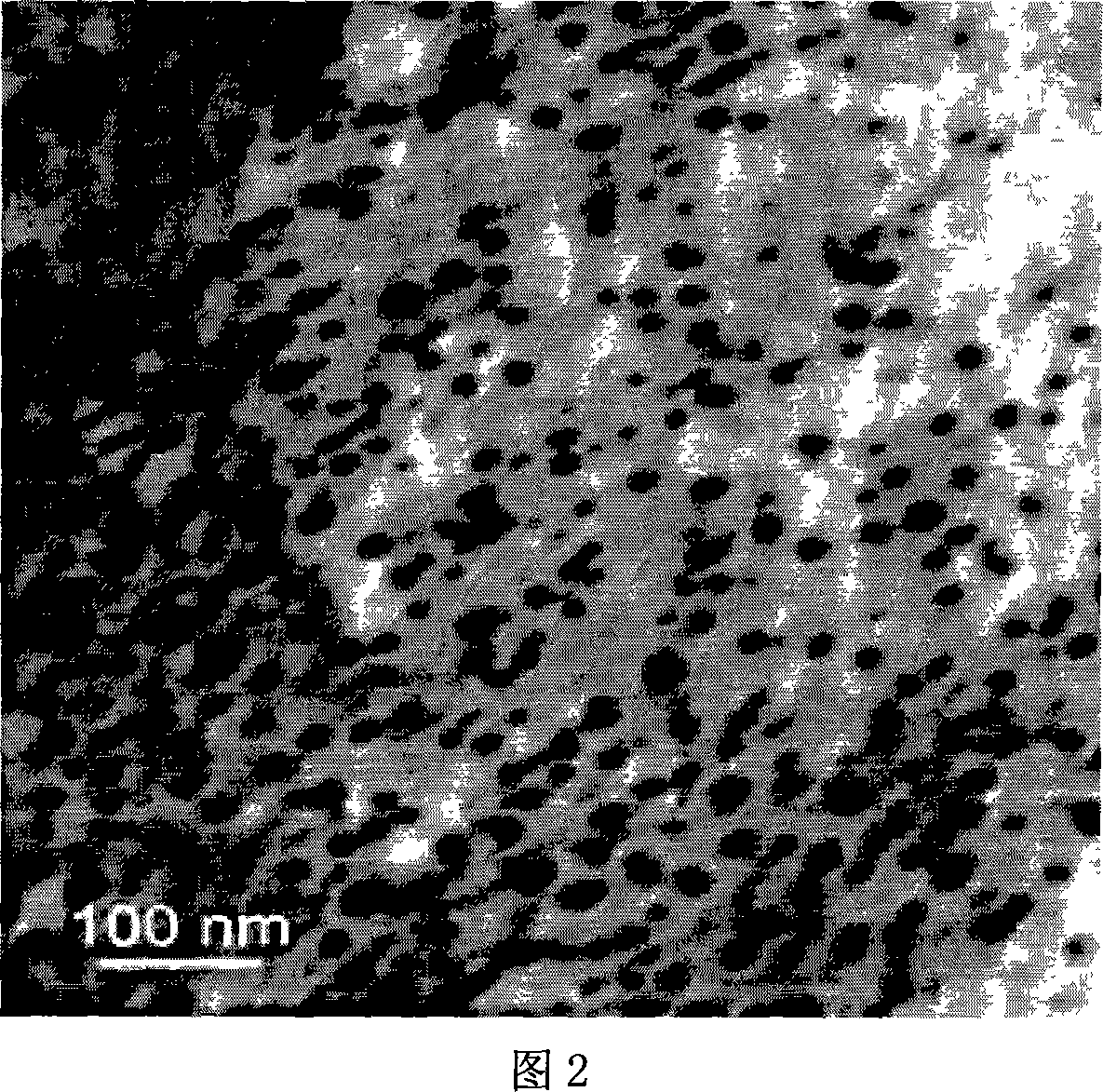
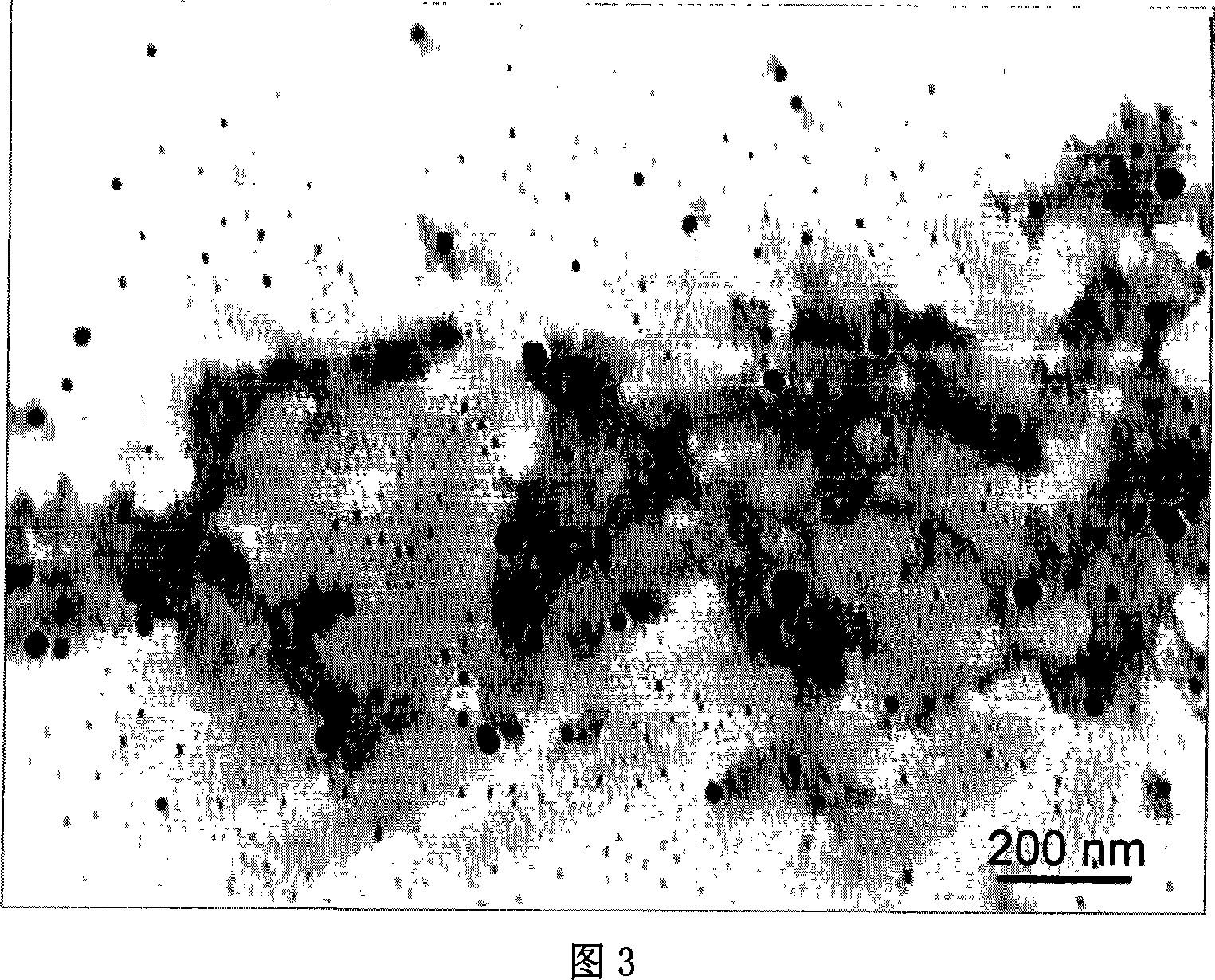
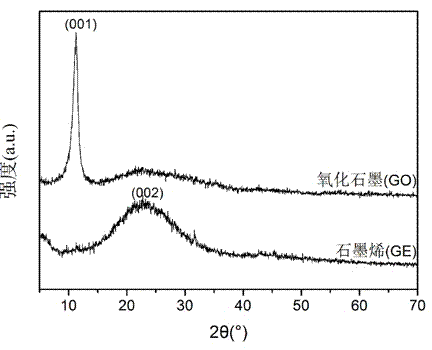
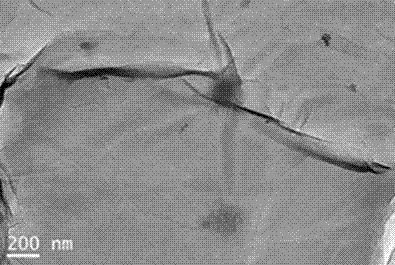
The invention belongs to the field of two-dimensional nano materials and preparation thereof, and particularly relates to a preparation method of graphene. The method is characterized by comprising the following steps: adding a reducer into a graphite oxide dispersion liquid, heating to react, and carrying out centrifugal separation to obtain the graphene, wherein the reducer is sodium diphenylamine sulfonate. The method has the advantages of more sufficient reduction of graphite oxide, easy product separation and simple purification process. In the preparation process, no stabilizer or dispersant is added, and the obtained graphene contains fewer impurity elements; and the invention has the characteristics of controllable process, short period, atmospheric conditions, simple equipment and excellent graphene properties, is suitable for industrial production, and has wide application prospects in the technical fields of catalysis, energy transfer and storage, sensors and the like.
Owner:苏州汇涵医用科技发展有限公司
Method for measuring total iron
InactiveCN104459017ANo dischargeReaction is easy to controlChemical analysis using titrationTest sampleWater chlorination
The invention relates to a method for measuring total iron, and belongs to the field of analysis and detection. The method comprises the following steps: S1, fusing an iron-containing test sample at high temperature by using alkali, and then leaching by using acid; S2, heating a leaching solution to be nearly boiled, dropping tin chloride (50-150g / L) to until the light yellow color appears, and cooling; S3, dropping a titanium trichloride solution until the tungsten blue color appears by taking sodium tungstate as an indicator; S4, dropping several drops of copper solutions, and shaking to fade the tungsten blue color; S5, adding a right amount of sulfuric-phosphorous mixed acid (2+3), and titrating until the purple color as an end point appears by taking sodium diphenylamine sulfonate (4-10g / L) as an indicator and using a standard potassium dichromate solution. The method disclosed by the invention substitutes that the traditional total iron measurement in which mercuric chloride needs to be applied, so that the environmental pollution is reduced, the reaction is easily controlled by adding the copper solution and mixed sulfuric acid, end point discoloration is relatively obvious, and the method is relatively accurate, fast, simple and convenient.
Owner:工信华鑫科技有限公司
Analytic method for measuring content of each component in sulfuric acid and nitric acid mixture
InactiveCN104360010AAccurate measurementHigh sensitivityChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorPhysical chemistryAniline
The invention discloses an analytic method for measuring the content of each component in a sulfuric acid and nitric acid mixture. The method comprises the following steps: firstly measuring the total acidity of the mixture by adopting an acid-base titration method; subsequently measuring the content of nitric acid by adopting an oxidation-reduction titration method, wherein discoloration sensitivity is improved by adding an auxiliary indicator such as sodium diphenylaminesulfonate so as to correctly judge a titration end point; finally calculating the difference value to obtain the content of the sulfuric acid. The method has the advantages of simple detection requirements and conditions, low detection cost, high sensitivity, accurate and reliable results and the like.
Owner:JIANGXI NORMAL UNIV
Method for measuring iron content of lithium iron phosphorous oxide iron source raw material of lithium ion power battery anode material
InactiveCN102590203AGuaranteed purityFade slowlyMaterial analysis by observing effect on chemical indicatorLithium oxidePower battery
The invention relates to a method for measuring the iron content of a lithium iron phosphorous oxide iron source raw material of a lithium ion power battery anode material. The method comprises the following steps: placing an iron salt raw material into a muffle furnace for high-temperature calcinations; heating and dissolving a calcined product by using concentrated hydrochloric acid; adding a proper amount of deionized water to adjust the acidity of a solution; continuously heating the solution till boiling; adding a few drops of methyl hesperidin indicators; adding a high-concentration stannous chloride solution until the solution becomes straw yellow; slowly and dropwise adding a low-concentration stannous chloride solution until the solution becomes pink; and shaking uniformly to obtain a colorless transparent solution; quickly cooling; adding a proper amount of sulfur-phosphorus mixture acid; dropwise adding a few drops of sodium diphenylaminesulfonate indicators; and titrating a potassium dichromate standard solution, and completing a reaction process when the solution is changed from blue-green into purple. The method is convenient to operate and easy to control in the whole process and accurate in the test result. Compared with the conventional method, the method has the advantages that mercury salt is not adopted, so the health of operation staff is not damaged, the environment pollution is avoided, and the method is suitable for testing of the lithium iron phosphorous oxide iron source raw material of the lithium ion power battery anode material.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Method for determining iron, aluminum and silicon in silica simultaneously
InactiveCN107543774AEasy to observeThe result is stableWeighing by removing componentChemical analysis using titrationSesquioxideEvaporation
The invention relates to a method for determining iron, aluminium and silicon in silica simultaneously. The method comprises the following steps that a silica sample is burned to reach the constant, hydrofluoric acid and nitric acid are added for dissolving, silicon is removed through evaporation to dryness and volatilization, and fluorine is expelled with nitric acid; burning is conducted to reach the constant at 1,050+ / -50 DEG C, and the difference between two weighing is the silicon dioxide quantity; residues obtained after silicon dioxide is determined is fused with a mixed solvent, leaching is conducted with diluted hydrochloric acid, a leaching solution is neutralized with ammonium hydroxide, high valence metal ions are made to form hydroxide precipitates, burning is conducted, and sesquioxide is generated and weighed; precipitates determining sesquioxide is fused with potassium pyrosulfate, after leaching and dissolving are conducted with hydrochloric acid, reduction is conducted with stannous chloride to reach light yellow, sodium tungstate serves as an indicator, residual ferric iron is reduced with titanium trichloride, at last, sodium diphenylamine sulfonate serves as anindicator, titration is conducted with a potassium dichromate standard titration solution, and the content of iron sesquioxide is measured. The determination method has the advantages of being shortin operation procedure, high in efficiency and stable and accurate in result.
Owner:BAOTOU IRON & STEEL GRP
Continuous determination method of sodium nitrite-potassium nitrate mixed salt in salt bath
ActiveCN101949859AAvoid "aging" phenomenonTimely adjustmentMaterial analysis by observing effect on chemical indicatorPotassium nitratePhosphoric acid
The invention relates to a continuous determination method of sodium nitrite-potassium nitrate mixed salt in a salt bath, which is mainly technically characterized by firstly heating sample solution of the sodium nitrite-potassium nitrate mixed salt to a certain temperature, using potassium permanganate standard solution for titration under appropriate acidity, and utilizing the quantitative reaction of sodium nitrite and the potassium permanganate standard solution to calculate the content of the sodium nitrite in the sample solution; further acidifying the sample solution, adding excess ferrous ammonium sulphate standard solution, heating till boiling, keeping for 2-4 minutes, cooing to normal temperature, then sequentially adding water, phosphoric acid solution and sodium diphenylamine sulfonate indicator solution, using the potassium permanganate standard solution for titration, obtaining the volume of the potassium permanganate standard solution consumed by the sample solution, simultaneously carrying out a blank test, obtaining the volume of the potassium permanganate standard solution consume in the blank test, and further calculating the content of potassium nitrate in the sample solution. The method can detect the change situation of components in the salt bath and prevent the components in the salt bath from aging, and has the characteristics of simple method, rapidness, low cost, high accuracy and the like.
Owner:江南工业集团有限公司
Active aluminum detection method
InactiveCN102937590AEasy to operateImprove test accuracyMaterial analysis by observing effect on chemical indicatorRoom temperatureSodium diphenylamine sulfonate
The invention discloses an active aluminum detection method. The method comprises the following steps of: preparing a Fe2(SO4)3 solution; weighing and dissolving the sample in the following specific processes of accurately weighing an aluminum powder sample in a container, and slowly adding the Fe2(SO4)3 solution prepared in the first step and 10% of a sulfuric acid solution into the container; instantly sealing an opening of the container and only leaving an outlet which is communicated with a NaHCO3 solution; quickly adding the NaHCO3 solution into the container, slightly swinging the container, and subsequently putting the container on a heat source to heat and boil till the reaction is complete; titrating the solution and calculating in the following specific processes of taking the container (namely a triangular flask) down, cooling the container to a room temperature, then dropwise adding a sodium diphenylamine sulfonate indicator into the container, and instantly titrating the solution by a K2Cr2O7 solution till the solution changes from green to purple or hyacinthine as a result; and then calculating the content of active aluminum by a formula. The active aluminum detection method has the beneficial effects of no need for purchasing special equipment, simple operation and high detection precision.
Owner:成都桑莱特科技股份有限公司
Novel method for determination of total iron of iron ore by potassium dichromate volumetric method
ActiveCN104111305AReduce pollutionNo significant differenceChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorIronstoneDissolution
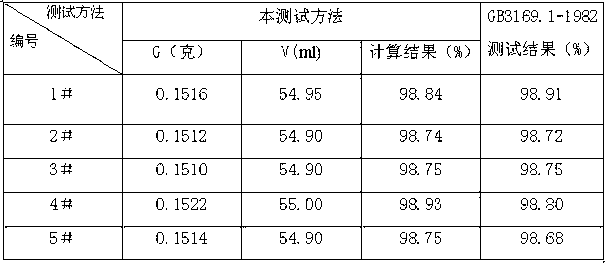
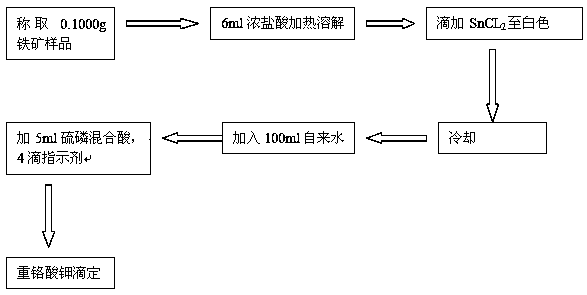
The invention discloses a novel method for determination of total iron of iron ore by a potassium dichromate volumetric method. The novel method comprises the following steps of crushing an iron ore sample to obtain powder having particle size less than 0.088mm, weighing 0.1000g of the crushed iron ore sample, putting the crushed iron ore sample into 250mL of a conical flask, adding 6mL of concentrated hydrochloric acid into the conical flask, carrying out heating dissolution, dropwisely adding a tin dichloride solution into the conical flask until the color is white, carrying out cooling, fast adding 100mL of tap water into the conical flask, adding 5ml of a sulfur-phosphor acid mixture standard solution into the conical flask, dropwisely adding 4 drops of a sodium diphenylaminesulfonate indicator into the conical flask, and carrying out titration by a potassium dichromate standard solution until the color is purple. The novel method reduces a reagent use amount by 1 / 2, is free of titanium trichloride and sodium tungstate and utilizes tap water to replace distilled water or deionized water. The novel method furthest reduces reagent and energy consumption and a lab test cost, reduces environmental pollution, reduces labor intensity of lab test workers, improves a lab test speed and reduces iron ore sample lab test time to about 3min from about 10min.
Owner:MAGANG (GROUP) HOLDING CO LTD +1
Method for determining ferric oxide in silica
InactiveCN107340359AMaster fastIncrease productivityChemical analysis using titrationPhosphoric acidDiiron Trioxide
The invention relates to a method for determining ferric oxide in silica. The method comprises the following steps: a silica sample is weighed and dissolved in sulfuric acid and phosphoric acid mixed acid, most ferric iron is reduced with stannous chloride in hydrochloric acid media, titanium(III) chloride is added dropwise to reduce residual ferric iron into ferrous iron with sodium tungstate as an indicator, and excessive titanium(III) chloride is oxidized with potassium dichromate; a potassium dichromate standard solution is used for titration with sodium diphenylamine sulfonate as an indicator; a blank control group test is performed, and the content of ferric oxide in silica is calculated. The method has the advantages that the operation is rapid and efficient and the result is accurate and stable.
Owner:BAOTOU IRON & STEEL GRP
Analysis method for chromium content in chromium iron alloy and silicon chromium alloy
InactiveCN105572118ALow costReduce lossMaterial analysis by observing effect on chemical indicatorBrickInterference factor
The invention discloses an analysis method for the chromium content in chromium iron alloy and silicon chromium alloy. The method comprises: weighing a sample, placing in a ceramic crucible containing sodium peroxide in advance, stirring fully and uniformly, covering on the surface with the sodium peroxide, keeping for 5min at 700 DEG C, taking out and slightly shaking the ceramic crucible; then placing on a refractory brick and cooling; after naturally cooling, placing the ceramic crucible in a beaker containing hot water in advance, adding H2SO4, and leaching inner fusant; after reaction is finished, drenching the ceramic crucible and lid in the beaker, and adding ammonium persulfate and the like; heating and boiling till a volume is 250ml, adding a NaCl solution, slightly boiling for a moment till while sediment occurs, and taking down and cooling to room temperature; adding phosphoric acid, water and sodium diphenylaminesulfonate indicator, and performing titration with ammonium ferrous sulfate till light green color occurs. By adopting the crucible technical solution, an experiment cost is reduced, and loss of noble metal is omitted; influences of other interference factors are avoided and a test analysis process becomes simple and fast.
Owner:WUHU XINXING DUCTILE IRON PIPES
Method for determination of iron in copper alloy
InactiveCN106404991AQuick responseEasy to observeChemical analysis using titrationPhosphoric acidSodium diphenylamine sulfonate
A method for determination of iron in a copper alloy comprises the following steps: (1) a copper alloy sample is weighed and put into a conical flask, and HCl and H2O2 are used for heating to dissolve the sample; (2) the copper alloy sample solution is taken and slightly cooled, an ammonia solution is used to adjust pH value to 6.8-7.2, the copper alloy sample solution is filtered and washed with the ammonia water until the copper alloy sample solution is free of copper ions, and hydrochloric acid is used for washing a precipitate into a conical flask; (3) tin dichloride is used for reduction of the solution to yellowish; then water is added, a mixed acid of phosphoric acid and sulfuric acid is added, a few drops of a sodium tungstate indicator are added, and a titanium trichloride solution is added dropwise until the solution is blue; then a potassium dichromate standard titration solution is used for titration of the solution until the blue color disappears; then three drops of a sodium diphenylaminesulfonate indicator are added, and the potassium dichromate standard titration solution is used for titration of the solution until a stable purple color as the end point is reached. The reaction speed is fast, the end point is stable, does not change color and is easy to observe, and the accuracy is good; meanwhile the determination process is short, determination efficiency is high, the determination method is easy to grasp, used chemicals are simple, and the method has the advantages of low pollution, low requirements for equipment and environment and low determination cost.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Volumetric analysis method adopting ascorbic acid to carry out reductometry on iron
InactiveCN103063668AEasy to operateLow assay costMaterial analysis by observing effect on chemical indicatorIronstonePhosphoric acid
The invention discloses a volumetric analysis method adopting ascorbic acid to carry out reductometry on iron. The method is characterized by dissolving an iron-containing sample by a conventional method, quantitatively reducing Fe<3+> to Fe<2+> with ascorbic acid, then oxidizing the excessive ascorbic acid with a 2,6-dichloroindophenol solution, in a sulfuric acid and phosphoric acid mixed environment, taking sodium diphenylaminesulfonate as an indicator and titrating Fe<2+> with a potassium dichromate standard solution until stable purple red appears to be the end point and computing the mass fraction of iron in the iron-containing sample. The method has the beneficial effects that a highly toxic mercuric chloride reagent is avoided and pollution is reduced; and the analysis method is easy to operate, has high accuracy and is suitable for determining the content of the major component iron in iron ores and iron powder.
Owner:长治学院
Method for detecting content of total iron in vanadium titanium powder
InactiveCN109342644AReduce usageMeet the needs of productionChemical analysis using titrationSodium diphenylamine sulfonateCopper sulfate
The invention provides a method for detecting content of total iron in vanadium titanium powder, characterized in that the detection method comprises following steps: (1) weighing a sample of the vanadium titanium powder to be detected and placing it in a container, adding sulfur and phosphorus mixed acid, and dissolving to obtain mixed solution; (2) adding concentrated hydrochloric acid to the mixed solution prepared in the step (1), boiling, dropping stannous chloride solution until pale yellow solution is obtained, using sodium tungstate solution as an indicator, dropping titanium trichloride solution until the solution turns light blue, and then dropping a drop of the titanium trichloride solution to obtain blue solution; (3) adding copper sulfate solution for catalysis to the blue solution prepared in the step (2) to obtain colorless solution; and (4) using sodium diphenylamine sulfonate solution as the indicator, dropping potassium dichromate standard solution to the colorless solution obtained in the step (3) until the solution turns light purple as an endpoint.
Owner:CHENGDE JIANLONG SPECIAL STEEL
Method for determining content of metallic magnesium in briquetting nodulizer through subtraction process
ActiveCN104133035AReduce pollutionSimplify analysis stepsWeighing by removing componentChemical analysis using titrationPhosphoric acidSodium diphenylamine sulfonate
The invention discloses a method for determining the content of metallic magnesium in a briquetting nodulizer through a subtraction process. The method comprises the following steps: dissolving metallic magnesium in a sample by dilute acetic acid, filtering, washing, drying a insoluble substance, and weighing; transferring a certain volume of a filtrate, completely reducing ferric iron to divalent iron by titanium trichloride with sodium tungstate as an indicator until tungsten blue is generated, oxidizing the above excess reducing agent by a dilute potassium dichromate solution, tiltrating divalent iron by a potassium dichromate standard solution in a sulfuric acid-phosphoric acid medium with sodium diphenylamine-sulfonate as an indicator, and calculating the mass fraction of iron in the filtrate; and subtracting the mass fraction of iron in the filtrate from the mass fraction reduced to the dissolving by the dilute acetic acid in the sample. The method has the advantages of simplicity, rapidness, high accuracy, high precision and the like, and fully meets the requirements of metallurgical furnace laboratory analysis.
Owner:INST OF RES OF IRON & STEEL JIANGSU PROVINCE
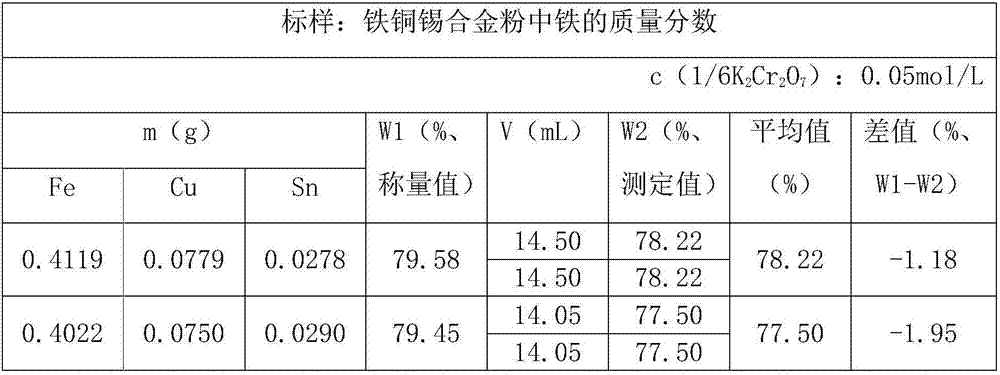
Measurement method for iron content in iron-copper-tin ternary prealloy powder
InactiveCN107462665ARapid determinationAccurate measurementChemical analysis using titrationSodium diphenylamine sulfonateFerric
The invention discloses a measurement method for iron content in iron-copper-tin ternary prealloy powder. In hydrochloric acid solution, Fe3<+> is recovered into Fe<2+> by using SnCl2, then HgCl2 is added to oxidize the excess SnCl2, sodium diphenylaminesulfonate is used as an indicator, and potassium dichromate standard solution is used for titration. The method provided by the invention can be quick in measurement, and accurate in measurement result.
Owner:泉州众志金刚石工具有限公司
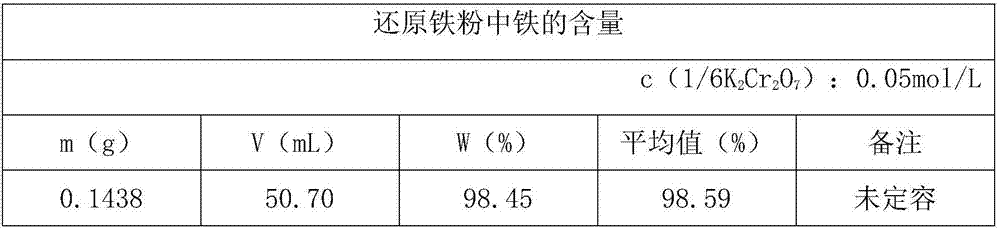
Calibration method of potassium dichromate standard solution
InactiveCN109580871AEasy to masterChemical analysis using titrationPhosphoric acidSodium tungstate dihydrate
The invention discloses a calibration method of a potassium dichromate standard solution. The calibration method comprises the following steps that S1, a pure iron sample is weighed and is placed in aconical flask, hydrochloric acid and concentrated nitric acid are added slowly; S2, the conical flask is boiled at low temperature, a stannous chloride solution and the hydrochloric acid are continuously added dropwise; S3, the conical flasks cools; S4, water and a sodium tungstate dihydrate solution are added in the conical flask, and a titanium trichloride solution is added dropwise; S5, the standard solution is added dropwise in the conical flask until blue disappears, sulphuric acid-phosphoric acid mixed acid is added immediately, and a diphenylaminesulfonic acid sodium salt indicator solution are added; S6, the potassium dichromate standard solution is titrated into the conical flask until the solution in the conical flask turns into blue-green from green, when the last drop shows astable purple, titration is completed, and the volume V of the potassium dichromate standard solution consumed by titration is recorded; and S7, according to the addition quantity, molar concentrationof the potassium dichromate standard solution can be calculated. The method is accurate in calibration, and is simple and easy to master.
Owner:JIUJIANG PINGGANG STEEL
Method for determining iron sesquioxide content in coal combustion improver
ActiveCN103439329AReduce dosageReduce analysis costsMaterial analysis by observing effect on chemical indicatorPotassium fluorideSesquioxide
The invention discloses a method for determining iron sesquioxide content in coal combustion improver, which comprises the following steps: dissolving a sample to be measured in mixed sulfuric acid and phosphoric acid with potassium fluoride used as cosolvent, fuming to a bottle neck, taking down, and cooling; adding hydrochloric acid, dropwisely adding stannous chloride, reducing to light yellow, heating, taking down, and cooling to 50-60 DEG C; dropwisely adding sodium tungstate, reducing to blue with titanium trichloride, and oxidizing with a potassium dichromate solution until blue disappears; dropwisely adding sodium diphenylamine sulfonate, and titrating with a potassium dichromate standard solution to stable purple (end point), thus obtaining the titer of the sample to be measured in milliliter; and calibrating the titer value of a standard substance of which the iron sesquioxide content is known, and obtaining the iron sesquioxide content in the sample according to the titer value and the titer of the sample in milliliter. According to the invention, no specific analytical apparatus is required; the method is convenient and quick to operate and low in analysis cost; and the determination result has favorable stability, reproducibility and accuracy. Thus, the method can satisfy determination of iron sesquioxide content in coal catalytic combustion improver for routine blast furnace injection.
Owner:WUKUN STEEL
Raspberry drop irrigation fertilizer
InactiveCN104761357APromote growthDoes not affect healthFertilizer mixturesSolubilityHazardous substance
The invention discloses a raspberry drop irrigation fertilizer which comprises the following components in percentage by mass: 15-28 percent of sodium diphenylamine sulfonate, 15-20 percent of auxiliary fertilizers, 1-3 percent of vitamins, 0.1-0.5 percent of amino acids, 2-4 percent of humic acid, 0.1-0.5 percent of alkaloid and the other components of purified water. According to the raspberry drop irrigation fertilizer disclosed by the invention, the defects that the conventional drop irrigation fertilizer adopts a single nutritional ingredient and contains harmful substances and drop irrigation equipment is easily blocked are overcome, and the advantages of comprehensive nutrition, no toxicity, high solubility and the effects of not blocking the pipeline and saving the production cost are realized.
Owner:平越
Method for measuring concentration of total-iron ion in chemical cleaning solution containing strong complex
InactiveCN109187849AEasy to operateHigh sensitivityChemical analysis using titrationPotassiumSodium diphenylamine sulfonate
The invention discloses a method for measuring the concentration of total-iron ion in chemical cleaning solution containing a strong complex. A principle is as follows: in an acidic condition, silversulphate is a catalyst; a strong complex substance is oxidized by ammonium persulfate and hydrogen peroxide, so that the complexation to iron ion is lost; furthermore, divalent iron is converted intoferric iron; ferric iron is reduced to divalent iron through stannous chloride; excess stannous chloride is oxidized by mercury bichloride; finally, sodium diphenylaminesulfonate is used as an indicator; and the reduced divalent iron is oxidized by potassium bichromate. The method is simple and rapid to operate and high in sensitivity and accuracy; measurement can be carried out by a conventionalanalysis vessel; the measurement time for one time is not beyond 25 min; the detection limit is 10 mg / L; the recovery rate is 92.5-107.3%; the detection range is 100-12000 mg / L; and test requirementsin a chemical cleaning field can be completely satisfied.
Owner:XIAN THERMAL POWER RES INST CO LTD +2
Continuous determination method of potassium nitrate and sodium nitrate mixed salt bath
InactiveCN106353218AThe determination method is simpleEasy to detectWeighing by removing componentAcetic acidPotassium nitrate
The invention discloses a continuous determination method of a potassium nitrate and sodium nitrate mixed salt bath. The method provided by the invention comprises the following steps: preparing a sample solution, determining and calculating the content of potassium nitrate, determining and calculating sodium nitrate and the like. In the acidic medium of acetic acid, sodium tetraphenylborate reacts with potassium nitrate to produce a potassium tetraphenylborate precipitate, filtering, drying and weighing are carried out, and then the content of potassium nitrate is calculated; in the presence of molybdenum salt, a concentrated sulfuric acid is used to acidify, the nitrate reacts with excess ammonium ferrous sulfate standard solution, sodium diphenylamine sulfonate is used as an indicator, a potassium dichromate standard titration solution is used for back titration, the total amount of the nitrate is calculated according to the amounts of the ferrous ammonium sulfate and the potassium dichromate standard titration solution, and the amount of potassium nitrate equivalent to sodium nitrate is subtracted to obtain the content of sodium nitrate. The determination method provided by the invention has the advantages of simplicity, convenient detection process, quickness, environmental protection, low cost, and accurate and consistent detection result, and provides support for the maintenance and quality control of potassium nitrate and sodium nitrate.
Owner:江南工业集团有限公司
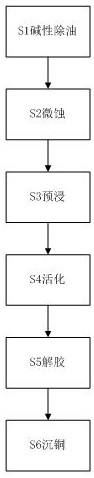
Chemical copper plating solution and method for plating copper substrate by using chemical copper plating solution
PendingCN113388829AImprove stabilityReduce porosityLiquid/solution decomposition chemical coatingPrinted element electric connection formationChemical platingCopper plating
The invention discloses a chemical copper plating solution and a method for chemical copper plating. The chemical copper plating solution comprises the components including, by mass concentration, a main salt, a reducing agent, a complexing agent, a stabilizer, an accelerator, a surfactant and the balance ultrapure water. The chemical copper plating solution further comprises a pH value regulator and an additive, wherein the additive is polyvinylpyrrolidone and sodium diphenylaminesulfonate. The chemical copper plating method comprises the steps of alkaline oil removal, micro-etching, preimpregnation, activation, peptization and copper deposition. Alkaline oil removal, micro-etching, preimpregnation, activation and peptization are pretreatment procedures to adsorb colloidal palladium particles to a PCB to be plated with copper. In the copper deposition process, copper ions are catalytically reduced to the PCB to be plated with copper through the chemical copper plating solution. In the chemical copper plating solution, the polyvinylpyrrolidone and the sodium diphenylaminesulfonate are used as the combined additive, so that the chemical copper plating solution has the characteristics of being environmentally friendly, low in price and easy to obtain, and the copper plating effect can be effectively improved while the copper plating rate is ensured.
Owner:惠州金晟新电子科技有限公司
Method for detecting organic matter in soil
InactiveCN101419175BImprove accuracyReduce edge effectsChemical analysis using titrationSoil organic matterOrganic matter
The invention relates to measurement for organic substances in soil, in particular to a method for measuring organic substances in soil. The method comprises the following steps: using a digestion instrument to replace a heating device; using a digestion tube to replace a conical flask to receive samples and reagent solution; covering a small funnel with a bent part above the digestion tube when heating; timing from boiling of the solution; taking down the digestion tube after 6 to 8 minutes; cooling the digestion tube; and using sodium diphenylamine sulphonate as indicator to titrate residual K2Cr2O4. The method has the advantages of good repeatability, high reproducibility, simplicity and practicability.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com