Measurement method for iron content in iron-copper-tin ternary prealloy powder
A technology of pre-alloyed powder and determination method, which is applied in the direction of chemical analysis by titration method, which can solve the problems of residual nitric acid and affecting the determination of the end point of reduction reaction titration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
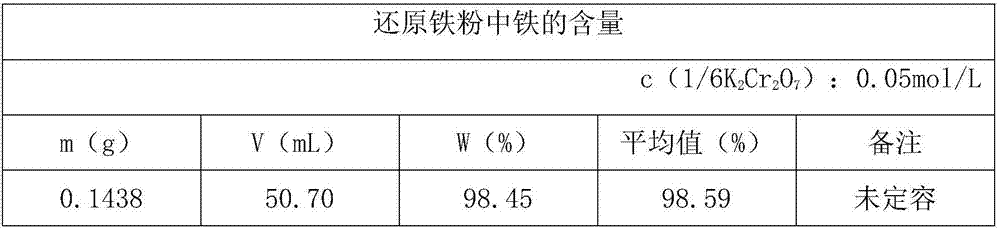
[0031] First, weigh 0.1000g of reduced iron powder into a 250mL Erlenmeyer flask, add 10mL of hydrochloric acid solution, 5mL of hydrogen peroxide, until the sample is completely dissolved, add 30mL of water, heat to boiling, remove excess hydrogen peroxide, drop while hot Add SnCl 2 solution until the solution is colorless and excess 1-2 drops.
[0032] Next, add 10mL HgCl 2 Saturated solution, mix well, let stand for 5min.
[0033] Finally, add 20mL of water, 20mL of sulfur-phosphorus mixed acid solution, 4-5 drops of sodium diphenylamine sulfonate indicator, and titrate with potassium dichromate standard solution. The solution turns from green to blue-purple as the end point.
[0034] Calculation formula:
[0035] In the formula:
[0036] ω(Fe)—mass fraction of iron in the sample;
[0037] c(1 / 6K 2 Cr 2 o 7 )—concentration of substance in potassium dichromate standard titration solution, mol / L;
[0038] V—the volume of potassium dichromate standard titration solu...
Embodiment 2
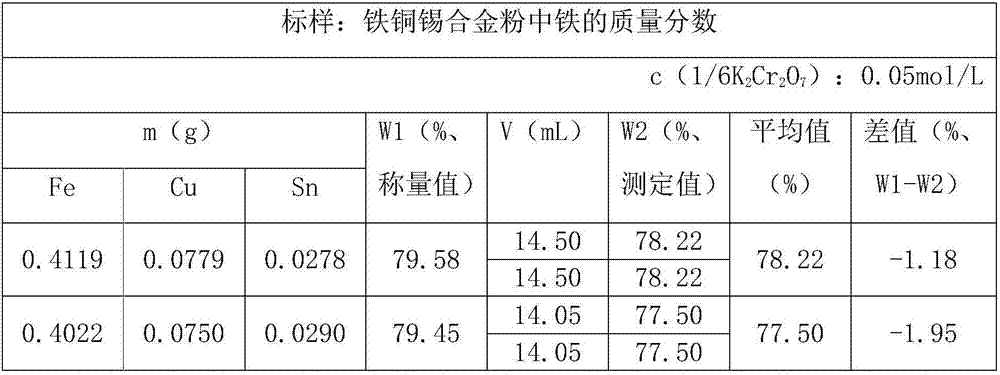
[0046] First, weigh 0.4000g of reduced iron powder, 0.0800g of elemental copper powder, and 0.0300g of elemental tin powder in the same 250mL conical flask, add 10mL of hydrochloric acid solution, 10mL of hydrogen peroxide, and wait until the sample is completely dissolved, add 30mL of water , heated to boiling, remove excess hydrogen peroxide, dropwise add SnCl 2 solution until the solution is colorless with 1-2 drops in excess.
[0047] Next, cool to room temperature, and dilute to a 100mL volumetric flask.
[0048] Next, pipette 10mL test solution, add 10mL HgCl 2 Saturated solution, mix well, let stand for 5min.
[0049] Finally, add 20mL of water, 20mL of sulfur-phosphorus mixed acid solution, 4-5 drops of sodium diphenylamine sulfonate indicator, and titrate with potassium dichromate standard solution. The solution turns from green to blue-purple as the end point. Calculation formula:
[0050] In the formula:
[0051] ω(Fe)—mass fraction of iron in the sample;
...
Embodiment 3
[0061] Step 2. Weigh 0.2000g of the powder to be tested in a 250mL Erlenmeyer flask, add 10mL of hydrochloric acid solution, 5mL of hydrogen peroxide, until the sample is completely dissolved, add 30mL of water, heat to boiling, remove excess hydrogen peroxide, while hot Add SnCl dropwise 2 solution until the solution is colorless and excess 1-2 drops.
[0062] Step 3, cool to room temperature, and dilute to a 100mL volumetric flask.
[0063] Step 4, pipette 10mL test solution, add 10mL HgCl 2 Saturated solution, mix well, let stand for 5min.
[0064] Step five, add 20mL water, 20mL sulfur-phosphorus mixed acid solution, 4-5 drops of sodium diphenylamine sulfonate indicator, titrate with potassium dichromate standard solution, the solution turns from green to blue-purple as the end point.
[0065] Calculation formula:
[0066] In the formula:
[0067] ω(Fe)—mass fraction of iron in the sample;
[0068] c(1 / 6K 2 Cr 2 o 7 )—concentration of substance in potassium dich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com