Method for measuring iron content of lithium iron phosphorous oxide iron source raw material of lithium ion power battery anode material
A technology for power batteries and positive electrode materials, which is applied in the direction of analyzing materials through chemical reactions and observing the impact on chemical indicators. It can solve human health hazards, mercury salt environmental pollution, and test accuracy. Poor and other problems, to achieve the effect of reducing pollution, accurate test results, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
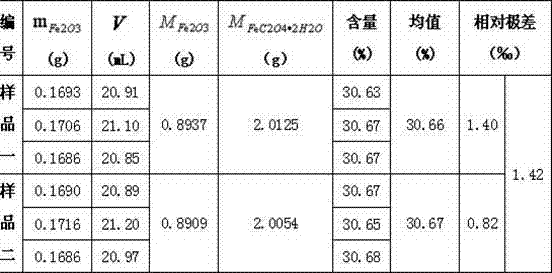
[0035] Example 1 : Weigh about 2.0125g of FeC with two crucibles 2 o 4 2H 2 O was placed in a muffle furnace, set the calcination temperature to 200°C, started heating, opened the furnace door after 2 hours, and weighed 1.0g KClO in a crucible 3 Put it into a muffle furnace, close the furnace door, adjust the calcination temperature to 600°C, and close the muffle furnace after calcination for 4 hours. When the furnace temperature drops below 200°C, the calcined product Fe 2 o 3 Place in a desiccator, cool to room temperature, and weigh the total weight to be 0.8937g. Weigh 0.1693g Fe2O3 sample into the conical flask, add 5mL concentrated hydrochloric acid, heat the conical flask slightly to ensure that the solution does not boil, after the Fe2O3 sample is completely dissolved, add 40mL deionized water to the conical flask, continue heating until boiling, Add 5 drops of methyl orange indicator, first use 10% SnCl2 reagent to titrate the color of the solution to light ye...
Embodiment 2
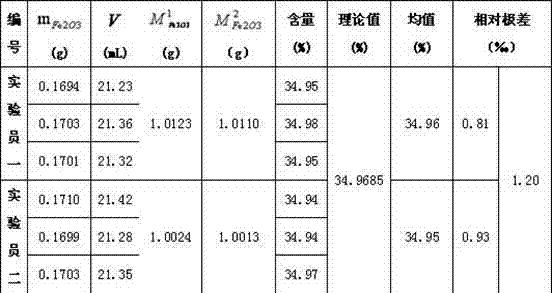
[0049] Example 2: Two experimenters weighed about 1.0123g of Fe in crucibles 2 o 3 Put the standard sample (99.99%) into the muffle furnace, set the calcination temperature to 200°C, start heating, open the furnace door after 2 hours, and weigh 0.5g KClO with a crucible 3 Put it into a muffle furnace, close the furnace door, adjust the calcination temperature to 600°C, and close the muffle furnace after calcination for 4 hours. When the furnace temperature drops below 200°C, the calcined product Fe 2 o 3 Place in a desiccator, cool to room temperature, and weigh the total weight to be 1.0110g. Weigh about 0.1694g Fe 2 o 3 Put the sample in the Erlenmeyer flask, add 5mL of concentrated hydrochloric acid, heat the Erlenmeyer flask slightly to ensure that the solution does not boil, and wait until Fe 2 o 3 After the sample is completely dissolved, add 40mL to the Erlenmeyer flask, continue heating until boiling, add 5 drops of methyl orange indicator, first use 10% SnCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com